
eBook - ePub
Disinfection By-Products in Water TreatmentThe Chemistry of Their Formation and Control
- 520 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Disinfection By-Products in Water TreatmentThe Chemistry of Their Formation and Control
About this book
Disinfection By-Products in Water Treatment describes new government regulations related to disinfection by-products. It explains the formation of microorganism by-products during water treatment and the methods employed to control them.The book includes several chapters on chlorine by-products and discusses techniques for the removal of chloroform from drinking water. It also describes gamma radiation techniques for removing microorganic by-product precursors from natural waters and the removal of bromate from drinking water.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Disinfection By-Products in Water TreatmentThe Chemistry of Their Formation and Control by Roger A. Minear,Gary Amy in PDF and/or ePUB format, as well as other popular books in Tecnología e ingeniería & Gestión medioambiental. We have over one million books available in our catalogue for you to explore.
Information
Part I
General Aspects
CHAPTER 1
Disinfection By-Products: Current Practices and Future Directions
Jeffrey L. Oxenford
Introduction
Drinking water utilities are preparing for major changes due to the proposed regulations for disinfection by-products (DBPs). The purpose of this chapter is to provide a prospective on the current practices and future research directions.
Background and Regulatory Requirements
Trihalomethanes (THMs) were first reported in drinking water in 1974.1,2 This was of great concern, since chlorination—which has been used since the early 1900s—had been the major weapon for preventing waterborne disease. In 1979, a maximum contaminant level (MCL) was established for total THMs (TTHMs) at 100 μg/l.3 The MCL was based on the annual average of four quarterly samples.
In 1981, the U.S. Environmental Protection Agency (EPA) published guidance for controlling THMs. For THM removal, aeration and activated carbon adsorption were discussed as effective technologies. Precursors to THM formation could be controlled by (1) oxidation by ozone or chlorine dioxide, (2) clarification by coagulation, precipitative softening, or direct filtration, or (3) adsorption by activated carbon. Other control technologies included oxidation with potassium permanganate, lowering the pH, or moving the point of chlorination to the end of the plant. Improvement of the source water quality was also mentioned, with the benefits of reducing precursors and chlorine demand.
The guidance document stated that THMs can be controlled by using alternative oxidants such as chloramines, chlorine dioxide, and ozone while maintaining the bacteriological quality of the water. Disadvantages of each alternative were discussed, such as ozone not leaving a residual, chloramines not being as effective for disinfection as chlorine and having toxicological properties, and the inorganic contaminants (chlorite and chlorate) associated with chlorine dioxide. The document also states but does not elaborate that other by-products may be produced by the alternative oxidants.
Table 1 Maximum Contaminant Levels and Maximum Residual Disinfectant Level Goals to Be Proposed In Stage 1 of the Disinfection/Disinfection By-Products Rule4

Beginning in 1992, water utilities, environmental groups, and EPA began regulatory negotiations on disinfection and DBPs. Due to the complexity of the issue and the amount of information still needed, regulations will be proposed in two stages.
Stage one will be proposed in 1994 and will provide MCLs for four classes of compounds and maximum residual disinfectant goals for chlorine, chloramines, and chlorine dioxide.4 Table 1 lists these requirements. Enhanced coagulation and granular activated carbon (GAC) were proposed as the best available technologies for precursor control.
The regulation will be revisited in 1998. It is anticipated that MCLs will be lowered for the THMs and haloacetic acids (HAAs). MCLs may also be developed for other by-products.
To provide the information necessary for the second stage, the information collection rule (ICR) has been proposed.5 The rule will require utilities serving a population greater than 10,000 to begin monitoring for microbial contaminants and DBPs. Surface water utilities serving a population greater than 100,000 and possessing raw water total organic carbon (TOC) exceeding 4 mg/l and groundwater utilities serving greater than 50,000 people with a finished water TOC of greater than 2 mg/l must test GAC and membrane treatment at the bench or pilot scale.
Another regulation that will play a significant role in the DBP issue is the surface water treatment rule (SWTR)4 This rule will require utilities to ensure adequate safeguards from microbial contamination. Components of the rule include filtration requirements for surface water facilities and disinfection capabilities to ensure 99.9% removal of parasites and 99.99% removal of viruses. This rule, combined with the DBP rule, brings a difficult challenge to utilities—ensuring disinfection while minimizing the DBPs produced.
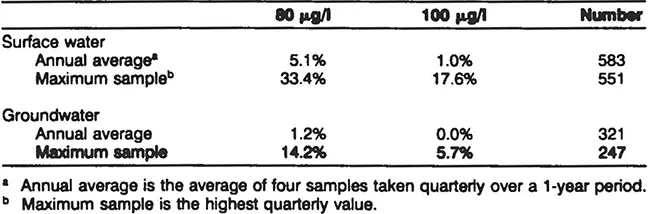
Table 2 Percentages of Utilities Exceeding Total THM Levels of 80 and 100 μg/l from the WIDB
Current Water Quality
The statistics provided in this paper were developed from the Water Industry Database (WIDB). The database was developed by the American Water Works Association Research Foundation (AWWARF) and the American Water Works Association (AWWA) and contains data from 1128 utilities that serve populations greater than 10,000. Data included in the database accounts for systems serving approximately 50% of the U.S. population. However, because the majority of all systems serve less than 10,000, the WIDB only accounts for 2% of the total community water systems. Data were collected from 1989 to 1992. New regulations and treatment modifications to meet future rules may impact the utility practices since the survey. This is important to keep in mind when reviewing the data presented in this paper.
The percentages of utilities exceeding the 100 μg/l MCL are given in Table 2. Approximately 1 % of the 583 surface water utilities had THM values exceeding 100 μg/l (the current MCL) based on the annual average of four quarterly samples. No groundwater utilities exceeded this value. When looking at the individual quarterly samples, 17.6% of the surface water and 5.7% of the groundwater facilities had at least one quarterly sample exceeding 100 μg/l. If the THM MCL is lowered to 80 μg/l, as is proposed in the stage-one regulations, data from the WIDB indicate that approximately 5% of the surface water and 1.2% of the groundwater facilities would need to reduce their THMs.
In the WIDB only 20% of the surface water and 11% of the groundwater facilities reported TOC data (Table 3). Of those reporting data in the WIDB, the average raw water TOC was 4.7 mg/I in surface water and 3.4 mg/l in groundwater. The median value for groundwater was 0.78 mg/l and for surface water was 3.9 mg/l. These values are somewhat higher than may be expected. One possible reason is that the facilities that are monitoring TOC are monitoring because they expect high levels of TOC. Amy et al.,6 in a recent survey of 100 sites (both groundwater and surface water) found the average TOC to be 2.73 mg/l and the median to be 2.07 mg/l. Estimates during the development of the ICR proposal7 were that 220 surface water utilities and 33 groundwater utilities would be required to evaluate treatment options to remove TOC.
Table 3 Average and Median TOC Values Reported In the WIDB

Industry Practices
Figure 1 shows the common points for oxidant addition at a water utility. The following sections will identify the common points of oxidant addition and reasons for their use.
Preoxidation
Oxidant may be added in transmission lines prior to entering a treatment facility for taste and odor control, to get a head start on disinfection, and to minimize biological growth in the line. Length of transmission lines may vary from relatively short distances to many miles. Biological growth in the line can significantly reduce the carrying capacity. One organism in particular, the zebra mussel, has been affecting transmission lines in the Great Lakes and has reduced flows by as much as 50%.8 Oxidants, primarily chlorine, have been used for their control.
Oxidant is added during the rapid mix phase of conventional treatment (coagulation, flocculation, filtration) to achieve disinfection, taste and odor control, iron and manganese removal, to improve coagulation and filtration, as well as to keep biological growth from colonizing in treatment basins. New research suggests that oxidation prior to coagulation may be necessary for arsenic removal.9·10
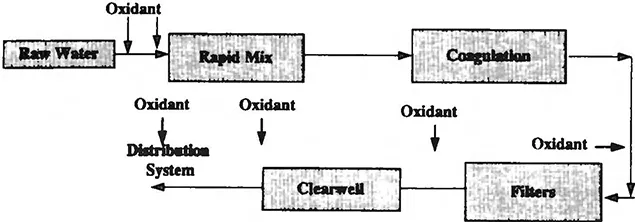
Figure 1 Possible points for addition of oxidant in a water treatment plant.
Table 4 Disinfection Practices of Surface Water Utilities From the WIDB
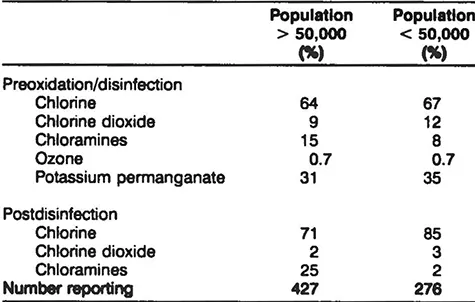
Table 4 shows the relative usage of preoxidants from the WIDB. For surface water facilities, approximately 65% of the surface water facilities use chlorine as a preoxidant. There is no significant difference in preoxidant usage between large- and medium-sized systems. The other major preoxidant is potassium permanganate which is used by over 30% of the surface water systems. There is no indication in the WIDB as to whether use of the preoxidant is seasonal or practiced continually. Intermittent oxidation is commonly used to control taste and odor and/or growth in the plant.
Intermediate Oxidation
Oxidant may be added prior to filtration to minimize biological growth on the filters, achieve additional disinfection, and oxidize iron and manganese. Oxidant may also be present in the filter backwash water. In the past, utilities in the U.S. have discouraged biological growth in filters. The concern has been over microorganisms passing into the product water. Numerous recent research projects including those by Glaze and Weinberg11 and Price12 have documented that biological treatment can be beneficial for reducing the byproducts of ozonation and for removal of natural organic matter (NOM).
Postdisinfection
Water leaving the filters is disinfected and usually stored prior to distribution. The concentration of the disinfectant and the amount of time held in storage is related to the disinfection effectiveness. Residual levels may also be boosted up at various points in the distribution system to ensure that a disinfectant residual is maintained. In the U.S., utilities are commonly required to maintain a residual to the furthest point in the distribution system.
Table 5 Disinfection Practices of Groundwater Utilities From the WIDB
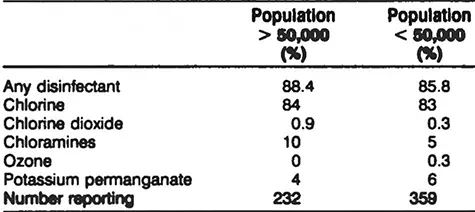
As shown in Tables 4 and 5, most utilities use chlorine as the postdisinfectant. Chloramines are used in approximately 25% of the large surface water utilities and to a much lesser extent by groundwater utilities and smaller surface water utilities.
By-Products Associated With Oxidant Use
By-Products Associated With Chlorine
Numerous by-products have been found w...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- PART I. GENERAL ASPECTS
- PART II. CHLORINE BY-PRODUCTS
- PART III. OZONATION AND BROMINATED DISINFECTION BY-PRODUCTS
- PART IV. CHLORAMINES AND CHLORINE DIOXIDE
- PART V. INFLUENCE OF NATURAL ORGANIC MATTER ON BYPRODUCTS
- Index