
- 328 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Antibodies in Diagnosis and Therapy
About this book
Monoclonal antibodies have had their impact on biomedical research for more than a decade. Beside their exuberant use as reagents, quite a number of diagnostic and therapeutic approaches have been followed and an impressive number of technological improvements, e.g., humanization, recombinant miniantibodies, have been elaborated to strengthen the principle. With respect to clinical applications, the first generation of antibody 'drugs' is yielding promising results while second and third generation antibody constructs are already underway. The book reviews the status of technological development and brings this into the perspective of clinical results. A rapidly growing amount of clinical data is collected in an expanding number of indications. Hence, the review of clinical study results has been grouped according to the fields of oncology and of chronic and acute inflammation. This book will be of interest to scientists working in the fields of oncology, immunology, internal medicine and clinical chemistry.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
MedicineSubtopic
Immunology1. RECOMBINANT ANTIBODIES: CONSTRUCTION AND PRODUCTION
MELVYN LITTLE and SERGEY M. KIPRIYANOV
German Cancer Research Center (DKFZ), Im Neuenheimerfeld 280, 69120 Heidelberg, Germany
INTRODUCTION
Antibodies are capable of highly specific interactions with a wide variety of ligands including tumor-associated markers, viral coat proteins and lymphocyte cell surface glycoproteins. They are therefore potentially very useful agents for the diagnosis and treatment of human diseases (see review by Riethmüller et al., 1993). However, the use of rodent antibodies for therapy poses a number of problems. One of these is the immunogenicity of monoclonal antibodies. Repeated doses of rodent antibodies elicit an anti-immunoglobulin response, referred to as HAMA (human anti-murine antibody; Jaffers et al., 1986; Khazaeli et al., 1994). The use of human monoclonal antibodies would alleviate this problem but only a restricted range of specificities are available. Furthermore, human hybridomas are often unstable and/or poor producers, thus making the production of large amounts of the human immunoglobulin very difficult (James, 1994).
In the past few years, some of the limitations of monoclonal antibodies as therapeutic agents have been addressed by genetic engineering. Such an approach is particularly suitable because of the domain structure of the antibody molecule, where functional domains carrying antigen-binding activities (Fabs or Fvs) or effector functions (Fc) can be exchanged between antibodies (Figure 1). On the basis of sequence variation, the residues in the variable domains are assigned either to the hypervariable complementarity-determining regions (CDRs) or to framework (FR) regions (Wu and Kabat, 1970). It is possible to replace much of the rodent-derived sequence of an antibody with sequences derived from human immunoglobulins without loss of function. This new generation of “chimeric” and “humanized” antibodies represents an alternative to human hybridoma derived antibodies and should be less immunogenic than their rodent counterparts. Furthermore, genetically truncated versions of the antibody may be produced ranging in size from the smallest antigen-binding unit or Fv through Fab′ to F(ab′)2 fragments. To stabilize the association of the recombinant VH and VL domains, they have been joined in single-chain Fv (scFv) constructs with a short peptide linker (Huston et al., 1988; Bird et al., 1988). More recently it has become possible to produce totally human recombinant antibodies derived from antibody libraries or single immune B cells.
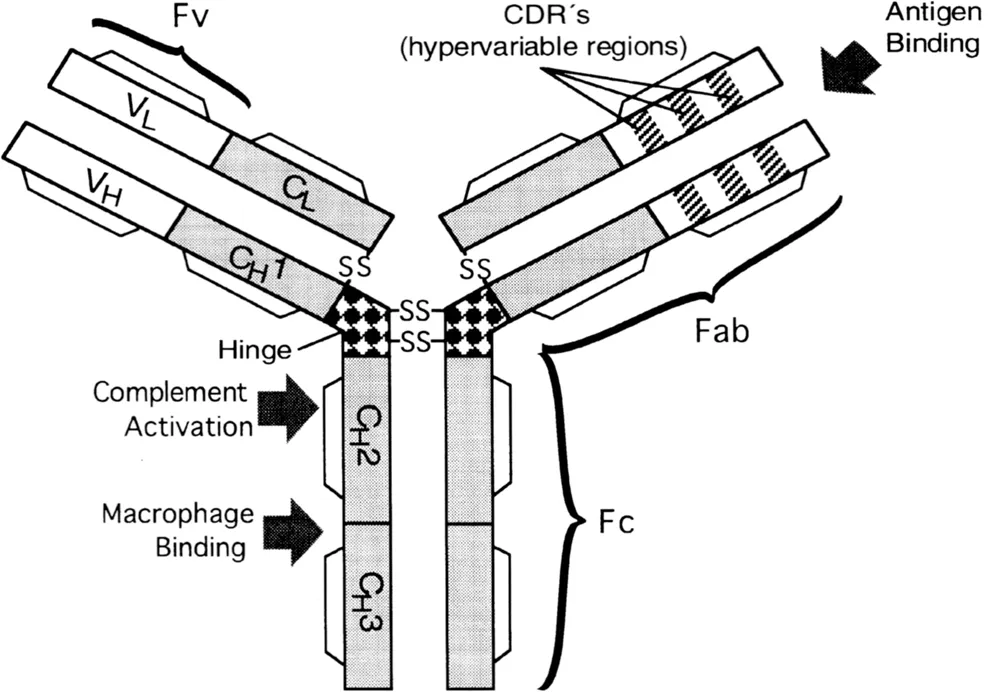
Figure 1 Schematic representation of the domain structure of an IgG molecule.
CLONING THE ANTIBODY VARIABLE REGIONS
Significant advances have been made in the in vitro immunization of human B cells (Borrebaeck et al., 1988) and in the development of transgenic mice containing human immunoglobulin loci (Brüggemann et al., 1989, 1991; Sarvetnick et al., 1993; Davies et al., 1993; Fishwild et al., 1996). Recombinant DNA technology can also be employed to generate human monoclonal antibodies from human lymphocyte mRNA. The genetic information for antibody variable regions is generally retrieved from a hybridoma-derived cDNA library by hybridization with oligonucleotides that bind to the constant domain genes (Adair et al., 1994; Pulito et al., 1996) or from total cDNA preparations using the polymerase chain reaction (PCR) with antibody-specific primers (Orlandi et al., 1989; Dübel et al., 1994). The PCR fragments can be directly sequenced and/or ligated into the appropriate expression vector. However, problems are often encountered when attempting to retrieve the correct V region genes from the hybridoma cDNA. For example, a cell line that was monoclonal in terms of secreted antibody proved to be oligoclonal in terms of mRNA species (Jiang et al., 1994). Furthermore, hybridomas in which the immortalizing fusion partner is derived from MOPC-21 may express a VL kappa transcript that is aberrantly rearranged at the VJ recombination site, and which therefore encodes a non-functional light chain (Cabilly and Riggs, 1985; Carroll et al., 1988). Cellular levels of this transcript may exceed that generated from the productive VL gene or the primer may be more homologous to its cDNA, so that a large proportion of the PCR product may not encode a functional light chain. Some errors can also be introduced by PCR itself.
Single bacterial colonies expressing antigen-specific antibody fragments can be identified by colony screening using antigen coated membranes (Dreher et al., 1991). The appropriate VH/VL combination may also be selectively enriched from an scFv phage library through a series of immunoaffinity steps referred to as “library panning” (McCafferty et al., 1990; Marks et al., 1991b). However, for these two approaches it is necessary to have sufficient amounts of soluble antigen for screening and for characterization of the selected clones by ELISA. In most cases, for antibodies directed against cell surface molecules, e.g. CD (cluster of differentiation) antigens, bio-panning and filter-screening procedures are not suitable.
In an attempt to circumvent the problems outlined above, a screening procedure for a hybridoma-derived scFv against the human transferrin receptor (TFR) was proposed (Nicholls et al., 1993b). The method is based on the specific cytotoxicity of scFv fused to a binding-deficient form of Pseudomonas exotoxin A (PE40). Small amounts of fusion protein were expressed in a rabbit reticulocyte lysate system and the specific toxicity was checked on a eukaryotic cell line expressing the TFR (Nicholls et al., 1993a,b). However, this protein synthesis inhibition assay only provides a qualitative assessment of antigen binding for a limited subset of scFv (i.e. scFv directed against a cell surface antigen that becomes internalized).
Another system for the rapid evaluation of functional antibody fragments directed against cell surface antigens employed cytotoxic bispecific antibodies (Sanna et al., 1995a). DNA coding for Fab fragments was fused to the gene for Staphylococcus aureus protein A (SpA) and expressed in E. coli. These Fab-SpA fusions were then mixed with a T-cell activating antibody specific for CD3 to form a bispecific cytotoxic complex. On the basis of an in vitro cell cytotoxicity assay, one could assess the antigen binding activity of the antibody fragment. This system is, of course, not suitable for antibody fragments specific for T-cell surface molecules.
Recently, we developed a rapid procedure for testing the function of hybridoma-derived antibody fragments. The method is based on a flow cytometric analysis using a fluorescence activated cell scanner (FACScan) of single-chain Fv molecules produced in small scale E. coli cultures binding to eukaryotic cells (Kipriyanov et al., 1996b). A simple extraction of the soluble periplasmic content from 5 ml cultures yields sufficient amounts of antibody fragment for an evaluation of its cell-specific binding. Constructs that have incorporated the wrong VL kappa transcript are readily identified and the scFv can be tested for functional binding without investing considerable time and effort in large scale expression. The proposed procedure was successfully used for cloning a scFv specific for human CD 19 on the surface of B-lymphocytes.
A simple procedure for the generation of human antibody fragments directly from single B cells or B-cell clones was recently described (Lagerkvist et al., 1995). The procedure is based on the antigen-specific selection of single human B cells using antigen-coated magnetic beads and a cellular amplification step followed by PCR amplification of V-region genes. Using this approach, it is possible to avoid the cumbersome hybridoma technology and to obtain human antibody fragments with the original VH/VL pairing.
GENETICALLY ENGINEERED MONOCLONAL ANTIBODIES
Chimeric Antibodies with Human Constant Regions
The first generation of recombinant monoclonal antibodies consisted of the rodent-derived variable regions fused to human constant regions. It is thought that the most immunogenic regions of antibodies are the conserved constant domains (Khazaeli et al., 1994). Because the antigen binding site of the antibody is localized within the V regions, the chimeric molecules retain their binding affinity for the antigen and acquire the function of the substituted C regions. For example, mouse: :human IgGl chimeric antibodies have been shown to mediate the lysis of tumor cells in the presence of human complement (Liu et al., 1987).
Clinical experience with rodent monoclonal and mouse::human chimeric antibodies indicates that for some antibodies a strong anti-idiotypic reaction is elicited. For example, 60% of the renal allograft recipients treated with antihuman CD3 murine mAb OKT3 developed antiidiotypic antibodies (Jaffers et al., 1986). Therefore, to reduce the murine content even further, procedures have been developed for humanizing the Fv regions.
Humanization by CDR Grafting
CDRs build loops close to the antibody’s N-terminus, where they form a continuous surface. Crystallographic analysis of several antibody/antigen complexes and other studies have shown that antigen binding mainly involves this surface (although some framework residues have also been found to be involved in the interaction with antigen). Thus, the antigen-binding specificity of an antibody is mainly defined by the topography of its CDR surface and by the chemical characteristics of this surface; these in turn are determined by the conformation of the individual CDRs, by the relative disposition of the CDRs, and by the nature and disposition of the side chains of the amino acids comprising the CDRs (Padlan, 1994).
A large decrease in the immunogenicity of an antibody was achieved by grafting only the CDRs of xenogenic antibodies onto human framework and constant regions (Jones et al., 1986; Verhoeyen et al., 1988). However, CDR grafting per se may not result in the complete retention of antigen-binding properties. Indeed, it is frequently found that some framework residues from the original antibody need to be preserved in the humanized molecule if significant antigen-binding affinity is to be recovered (Queen et al., 1989; Co et al., 1991; Ohtomo et al., 1995).
Two major approaches have been used to produce effective CDR-grafted antibodies (Co and Queen, 1991). In one approach (Queen et al., 1989), human V regions showing the greatest sequence homology to murine V regions are chosen from a database in order to provide the human framework. The selection of human FRs can be made either from human consensus sequences or from individual human antibodies (Kolbinger et al., 1993). In some rare examples, simply transferring CDRs onto the most identical human V-region frameworks is sufficient for retaining the binding affinity of the original murine mAb (Roguska et al., 1996). However, in most cases, the successful design of high...
Table of contents
- Cover
- Half Title
- Series Page
- Title Page
- Copyright Page
- Table of Contents
- Preface to the Series
- Preface
- Contributors
- 1 Recombinant Antibodies: Construction and Production
- 2 Bispecific Antibodies
- 3 Targeted Cytotoxicity: Antibody–Drug and Antibody-Toxin Conjugates
- 4 Selective Drug Delivery Using Targeted Enzymes for Prodrug Activation
- 5 Immunoscintigraphy and Radioimmunotherapy
- 6 Clinical Studies in Oncology
- 7 Clinical Studies in Acute and Chronic Inflammation
- 8 Marrow Purging and Stem Cell Preparation
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Antibodies in Diagnosis and Therapy by Matzku in PDF and/or ePUB format, as well as other popular books in Medicine & Immunology. We have over one million books available in our catalogue for you to explore.