
- 376 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Control of Polymerization Reactors
About this book
This reference and text provides an in-depth description of developments in control techniques and their application to polymerization reactors and offers important introductory background information on polymerization reaction engineering.;Discussing modelling, identification, linear, nonlinear and multivariable schemes, Control of Polymerization Reactors: presents all available techniques that can be used to control reactors properly for optimal performance; shows how to manipulate pivotal variables that affect reactor control; examines methods for deriving dynamic process models to improve reactor efficiency; reviews reactor control problems and points out end-use properties; supplies methods for measuring process variables, and ways to estimate variables that can't be measured; and explains how single-input, single-output (SISO) strategies can be effectively used for control.;Filled with illustrative examples to clarify concepts, including more than 730 figures, tables and equations, Control of Polymerization Reactors is intended for use as a reference for chemical, process development, process design, research and development, control systems, and polymer engineers; and polymer chemists and physicists; as well as a text for upper-level undergraduate and graduate students in polymerization reactor control courses.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Introduction to Polymerization
In this chapter the fundamentals of polymerization as they apply to control of polymerization reactors will be highlighted. Section 1.1 will give a very brief overview of polymeric structure. Sections 1.2, 1.3, 1.4 and 1.5 will present the four basic mechanisms of polymerization; a fundamental knowledge of polymer science will be presumed. For a good treatment of the subject, the reader may refer to the texts by Billmeyer [1], Rodriguez [2], Rudin [3], Williams [4] and Odian [5]. Structure-property relationships are well covered by Seymour and Carraher [6].
1.1 Macromolecules
This section will provide a starting point for the discussion of polymerization reactions and their control. The section is broken into three major topics: a brief description of polymer structures, a systematic definition of the molecular weight distribution (MWD), and a brief description of techniques of polymer analysis. An overview of polymer structures is included to motivate later discussions of control of polymer structure. The description of MWD serves to define the nomenclature for later use. The discussion of polymer analysis is meant to lead into later applications of on-line measurements of polymer properties. Recent advances in polymer characterization can be found in Ref. [7].
1.1.1 Strncture
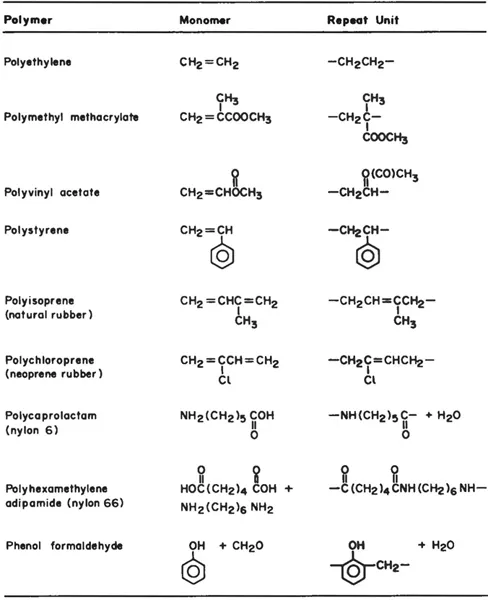
The concept of macromolecules is not an old one. Some polymeric products were commercially available in the late 1800s, mostly modified cellulose and other modified natural macromolecules, but an understanding of the nature of macromolecules paralleled the development of the first synthetic polymers in the early 1900s. A polymer is a large molecule made up of repeating units referred to as monomer units. Whereas each monomer unit may have a molecular weight of less than 100, when a polymerization reaction is carried out to join these monomer units, the molecular weight of the resulting polymer can be very high. Most commercial polymers have molecular weights between 10,000 and several million. Specialty polymers and biopolymers such as proteins may have molecular weights that are substantially higher. Examples of common monomers and their resulting polymers are given in Table 1.1.
The synthetic polymer industry began when H. L. Baekeland developed a polymer (Bakelite) resulting from the reaction of phenol and formaldehyde. Formaldehyde reacts with phenol under mild conditions to form a methylol derivative. This, then, forms the repeat unit and will take part in a condensation reaction, under acid conditions with excess phenol (“novolac” resins) or under alkaline conditions with excess formaldehyde (“resole” resins), to form polymers of molecular weights as high as 1000. This corresponds to about 10 repeat units per chain. The number of repeat units per chain is used as a specification of the length of the polymer chain, and is called the t:kgree of polymerization (x). The molecular weight of a given chain is then the degree of polymerization times the molecular weight of the repeat unit. Thus, for the Bakelite example, the molecular weight of the repeat unit is 106. If the degree of polymerization is 10, then the molecular weight of the polymer chain is 10 × 106 = 1060.
Much higher molecular weights are possible. For instance, if methylmetbacrylate (monomer) is polymerized in an emulsion system (Chapter 4), molecular weights of several million result. For a polymer (polymethylmethacrylate) molecular weight of 2,000,000 and a monomer (methylmethacrylate) molecular weight of 100, the average degree of polymerization must be 20,000. That is, there are 20,000 monomer units per polymer molecule. This example also illustrates a different mechanism of polymerization. As Table 1.1 indicates, the polymerization of methylmetbacrylate occurs, not by condensation, but by the opening of a double bond to form the polymer backbone.
Most polymerizations take place by one of two general mechanisms, step-growth or addition po/ymeri1plion. Step-growth takes place when functional groups on the monomer molecules react to form dimers or units containing two monomer molecules. This reaction is often a condensation reaction in which water or other low-molecular-weight by-products (HCI, CH3COOH, etc.) are formed, as in the reaction of phenol with formaldehyde to form a phenolic resin with water as a by-product. In the idealized case, the dimer units then react with other dimers to form tetramers. The tetramers then react to form larger molecules. The time to form a high-molecular-weight polymer chain may be of the order of hours. This will be important in the design of reactors for step-growth polymerization.
Table 1.1 Examples of common high polymers.
Addition polymerization takes place via the opening of a double bond on...
Table of contents
- Cover Page
- Half title
- Title Page
- Copyright Page
- Dedication
- Preface
- Contents
- 1 Introduction to Polymerization
- 2 Kinetic Analysis of Polymerization
- 3 Polymerization Reaction Engineering
- 4 Heterogeneous Polymerization
- 5 Framework of the Control Problem
- 6 Measurement and Estimation of Process Variables
- 7 Process Modeling and Identification
- 8 Single-Loop Reactor Control Strategies
- 9 Multivariable Control of Polymerization Reactors
- 10 Nonlinear Control Strategies for Polymerization Reactors
- 11 Introduction to Polymer Processing
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Control of Polymerization Reactors by Joseph Schork in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.