![]()
IV
Applications
12 Flame Retarded Polymer Foams for Construction Insulating Materials Zhengzhou Wang, Xiaoyan Li, and Lei Liu
Introduction
Flame Retardation of Rigid Polyurethane Foams
Flame Retardation of PS Foams
Toughening of Phenolic Foams
Conclusion and Perspectives
13 Recent Advances in Flame Retardant Textiles Bin Fei and Bin Yu
Introduction
Flame Retardation of Textile Materials
Recent Advances in FR Textiles
Summary and Outlook
14 Flame Retardant Polymer Materials Design for Wire and Cable Applications Christian Lagreve, Laurent Ferry, and Jose-Marie Lopez-Cuesta
Introduction
Standards and Tests for Cables
Main Polymers and Flame Retardants Used in the Cable Industry
Nanotechnology, Environmental Issues, and Future Trends
Conclusions
15 Flame Retardant Epoxy Resin Formulations for Fiber-Reinforced Composites Manfred Döring, Sebastian Eibl, Lara Greiner, and Hauke Lengsfeld.
Introduction
Influence of the Epoxy Resin Formulation on the Performance of Flame Retardants
Impact of Fiber Reinforcement on the Fire-Retardant Behavior of Epoxy-Based Composites
Selected Examples for Flame-Retardant Epoxy Resin Formulations for Fiber-Reinforced Composites
Formation of Respirable Fibers in Carbon-Fiber-Reinforced Epoxy Resins during Combustion and Prevention Thereof
![]()
12
Flame Retarded Polymer Foams for Construction Insulating Materials
Zhengzhou Wang, Xiaoyan Li, and Lei Liu
12.1 Introduction
12.2 Flame Retardation of Rigid Polyurethane Foams
Reactive-Type Flame Retardants
Additive-Type Flame Retardants
12.3 Flame Retardation of PS Foams
Flame Retardation of XPS Foams
Flame Retardation of EPS Foams
12.4 Toughening of Phenolic Foams
Chemical Toughening
Physical Toughening
12.5 Conclusion and Perspectives
12.1 Introduction
Polymer foams are composed of a polymer matrix incorporated with either gas bubbles or gas tunnels, with either a closed-cell or open-cell structure. Polymer foams have many advantages, for example, lightless, low thermal conductivity, easy process, and affordable cost, etc. and are widely used in many sectors of industry, e.g., building, transportation, furniture, chemical industry, and so on. Because the combustible volatile hydrocarbon liquids are often used as blowing agents and most polymer matrices themselves are also flammable, polymer foams, such as polystyrene (PS) foam and polyurethane (PU) foam, are easily ignited. Ignition of these polymer foamy materials is one of the most common reasons accounting for residential home fires and a large amount of fire civilian fatalities (Wang et al. 2014), such as the 2010 Shanghai high-rise apartment building fire (58 deaths) and the 2017 London Grenfell Tower fire (72 deaths). Therefore, flame retardation of PU and PS foams is generally needed in the building and transportation industry. The commonly used flame retardants for polymer foams include two categories: halogen-containing and halogen-free flame retardants. Halogen-containing compounds usually allow considerable improvements of flame retardancy of the foams. However, these kinds of flame retardants tend to release some toxic smoke and gases during fire. Due to environmental concerns, people pay more attention to halogen-free flame retardation of polymer foams.
Compared with PU and PS foams, phenolic foams (PF) have many advantages, such as good heat resistance, excellent flame retardancy, and low smoke and toxic gas release on burning. However, PF foams also have some disadvantages, such as brittleness and friability, which greatly limit their applications. The commonly used toughening agents for PF foams including ethylene glycol, polyethylene glycol (PEG), polyurethane pre-polymer, some elastomers, etc., however, may deteriorate the excellent flame retardancy of PF foams because those agents are highly flammable. Therefore, it is ideal for toughening agents to toughen PF foams without the cost of their excellent flame retardancy. Moreover, some solid fillers, i.e., fibers (glass and aramid fibers) and nano-materials (clays, carbon nanotubes, graphene) can increase the toughness of PF foams, generally without decreasing the flame retardancy of PF foams. For those solid fillers, especially nano-fillers, their uniform dispersion in the PF resin is a challenging task.
In this chapter, we will review the recent advances of flame retardation of rigid PU foams and PS foams, as well as toughening and flame retardation of PF foams.
12.2 Flame Retardation of Rigid Polyurethane Foams
PU foams are mainly divided into flexible polyurethane (FPU) foams and rigid polyurethane (RPU) foams, their market share of PU foams is about 48% and 28%, respectively (Singh and Jain 2009). FPU foams have been widely used in furniture cushioning and mattresses, and RPU foams have widespread applications in building, transportation, refrigeration industry, and so on (Al-Homoud 2005; Wu et al. 1999). PU foams are highly combustible; therefore, flame retardation of the foams is needed in most applications. A comprehensive review of the flame retardancy of polyurethanes including PU foams with emphasis on flame retardants in commercial use was published by Weil and Levchik (2004). Due to the environmental concerns of halogen-containing flame retardants, the development and application of halogen-free flame retardants for RPU foams has become a subject of extensive investigation.
The approaches for improving the fire retardancy of RPU foams consist of two categories: (1) reactive-type flame retardation, e.g., reactive flame retardant diols or polyols containing phosphorus and/or halogen, or containing phosphorus and/or nitrogen are generally used to react with isocyanates to prepare the flame retarded (FR) PU resin; (2) additive-type flame retardation, e.g., flame retardants are directly added by simple mechanical mixing at the compounding stage of PU foams (Levchik et al. 2004; Singh and Jain 2009). The additive-type flame retardants usually do not participate in the foaming reaction. These kinds of flame retardants can act as plasticizers if they are compatible with the polymer; otherwise, they are considered as fillers (Meng et al. 2009). As for the reactive-type fire retardants, they can build chemically into the PU molecule chain during the foaming reactions, and it is generally thought that greater permanence of flame retardancy can be maintained by this method. This part aims to review the recent developments regarding the use of halogen-free flame retardants for RPU foams.
12.2.1 Reactive-Type Flame Retardants
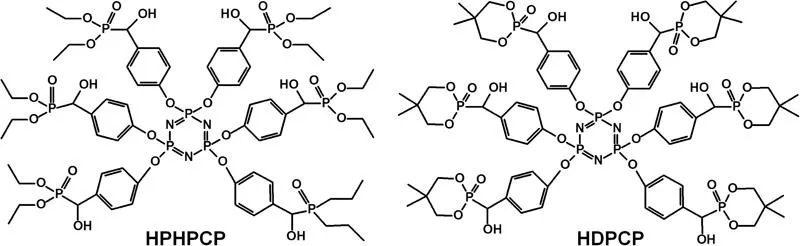
The non-halogenated reactive flame retardants are generally diols or polyols containing P, N, or P–N groups. The increased flame retardancy of phosphorus-containing RPU foams is attributed to the fact that phosphorus both promotes carbonization in the condensed phase and inhibits combustion in the gas phase. For instance, Wu et al. (2013) synthesized ethanolamine spirocyclic pentaerythritol bisphosphonate (EMSPB) and found that the RPU foam with 25 wt% EMSPB can pass the UL-94 V-0 rating with a limiting oxygen index (LOI) of 27.5%, however, the compressive strength and initial thermal stability of the FR RPU foams decrease compared to the pure RPU foam. Two reactive flame retardants, e.g., hexa-(phosphite-hydroxyl-methyl-phenoxyl)-cyclotriphosphazene (HPHPCP) (Figure 12.1) containing phosphazene and phosphate, and hexa-(5,5-dimethyl-1,3,2-dioxaphosphinane-hydroxyl-methyl-phenoxyl)-cyclotriphosphazene (HDPCP) (Figure 12.1) containing phosphazene and cyclophosphonate were synthesized, and it was observed that the LOI values of the RPU foams containing 20 wt% HPHPCP and 25% HDPCP are 26% and 25%, respectively (Yang et al. 2015, 2017). HPHPCP has a positive effect on compressive strength, while HDPCP has a negative influence on the strength of the RPU foams which may be because powdered HDPCP cannot react completely during the fast foaming process. Introduction of both HPHPCP and HDPCP resulted in an increase in the initial thermal decomposition temperature (T5%) and the residue of the FR RPU foams at high temperatures, which was attributed to the extent of enhancement of higher crosslinking due to the multifunctional reactive groups of the reactive flame retardants. Nevertheless, the incorporation of the two flame retardants, especially at their high loadings showed a negative influence on thermal insulation of the RPU foams. Liu et al. (2016) synthesized a melamine-based polyether polyol (HMMM–PG), and found that the physical, mechanical, and fire-retardant properties of RPU foams were improved due to the formation of a continuous and dense char layer.
FIGURE 12.1 Chemical structures of hexa-(phosphite-hydroxyl-methyl-phenoxyl)-cyclotriphosphazene (HPHPCP) and hexa-(5,5-dimethyl-1,3,2-dioxaphosphinane-hydroxyl-methyl-phenoxyl)-cyclotriphosphazene (HDPCP).
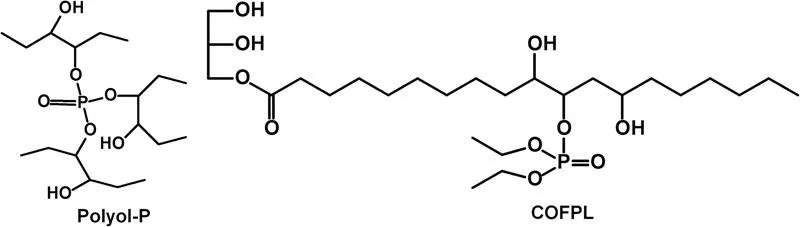
Recently, many researchers have paid attention to the modification of bio-based polyols to improve the mechanical and flame retarded properties of RPU foams. Cardanol has many applications in polymer chemistry, for example, in epoxy resins, phenol–formaldehyde resins, non-ionic surfactants, and polyurethanes. Ionescu et al. (2012) synthesized new bio-based Mannich polyols using cardanol and introduced the new Mannich polyols into RPU foams. It was found that both physico-mechanical and fire retardant properties of RPU foams were improved. Hydroxylated vegetable oils, whether synthesized or natural ones (castor oil), have been used to prepare polyurethanes through the reaction of hydroxyl groups with isocyanates. A phosphorylated polyol (Polyol-P, Figure 12.2) derived from vegetable oils was successfully synthesized, and its effect on the flame retardancy of RPU foams was investigated. RPU foams with densities between 30 and 39 kg/m3 demonstrated elliptical shaped closed-cells and homogeneous size distribution, due to the fact that phosphorylated polyols are made up of phosphate mono and diesters (Heinen et al. 2014). It was reported that the LOI of the RPU foams prepared with a phosphorus-containing castor oil-based flame-retardant polyol (COFPL, Figure 12.2) reached to 24.3% without any other flame retardant, and the compression strength of the modified RPU foams was improved (Zhang et al. 2014). The SEM images showed that the overall cell structure of the COFPL modified foams became more uniform, the cell walls became thinner, and the amount of broken cells decreased.
FIGURE 12.2 Structure of phosphorylated polyols (Polyol-P) and castor oil based flame-retardant polyols (COFPL).
In addition, there are some publications on RPU foams modified by boron-containing diols or polyols. Different boron-containing triols were synthesized from the reaction of boric acid with diols such as 1,2-propanediol, 1,3-butanediol or with aminoalcohols such as monoethanol-amine (Czupryński and Paciorek 1999; Czupryński et al. 2002). The incorporation of tri(hydroxyetylamine) borate obviously improved the flame retardancy of RPU foams, but a decrease in the brittleness of the foams was observed (Czupryński et al. 2002). Paciorek-Sadowska synthesized organic polyols based on boric acid and di(hydroxymethyl)urea derivatives, and their results showed that the boron- and nitrogen-containing polyols effectively increased fire resistance of RPU foams (Paciorek-Sadowska et al. 2015a, 2015b).
The reactive-type flame retardant diols or polyols aforementioned are usually applied in combination with regular diols or polyols to prepare FR RPU foams. How the FR diols or polyols participate in the foaming process of RPU foams is still not quite clear. Moreover, the fire retardancy stringent requirements of FR RPU foams prepared by this method are generally difficult to meet (e.g., Fire Retardant Grade B1) in the building and construction industry.
To improve further flame retardancy of RPU foams, reactive-type flame retardants are often combined with other additive-type flame retardants like expandable graphite (EG). A phosphorus-containing polyol (BHPP) and a nitrogen-containing polyol (MADP) as shown in Figure 12.3 were synthesized through dehydrochlorination...