
- 608 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Catalyst Handbook
About this book
This book bridges the gap between theory and practice. It provides fundamental information on heterogeneous catalysis and the practicalities of the catalysts and processes used in producing ammonia, hydrogen and methanol via hydrocarbon steam reforming. It also covers the oxidation reactions in making formaldehyde from methanol, nitric acid from ammonia and sulphuric acid from sulphur dioxide. Designed for use in the chemical industry and by those in teaching, research and the study of industrial catalysts and catalytic processes. Students will also find this book extremely useful for obtaining practical information not available in more conventional textbooks.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
Physical SciencesSubtopic
Industrial & Technical ChemistryChapter 1
Fundamental Principles
1.1. Fundamentals of Heterogeneous Catalysis
1.1.1. Introduction
The word “catalysis” is used to describe many phenomena but in all instances an agent (the catalyst) exerts a more-than-proportional influence over some change. Indeed, the word catalysis, coined by Berzelius in 1836 to describe some enhanced chemical reactions,1 is now used popularly in a non-technical way. In this book we shall restrict use of the term “catalysis” to the heterogeneous catalysis of gas-phase reactions; that is, the promotion of reactions between gases by the use of solid catalysts. We shall be concerned mainly with a few reactions of great importance in the heavy inorganic chemicals industry. The simplicity of the overall chemistry of these reactions usually hides a bewildering complexity of separate reaction steps on the catalyst surface. The discussion in this chapter is limited to a few general principles, which are illustrated by examples from the processes described in later chapters. Further study, essential for any full understanding of heterogeneous catalysis, can be undertaken using the books listed in Appendix 1. All of the topics of this chapter are treated comprehensively in a recent book by Richardson1a.
Catalysts are frequently defined as materials which accelerate chemical reactions without themselves undergoing change. As the manager of any plant using a catalytic process knows, this is too optimistic a definition: the properties of all real catalysts do change with use. The definition is also unsatisfactory in a more general way, for it implies that the acceleration is brought about without direct involvement of the catalyst in the process. Rather than attempting to produce an alternative succinct definition, we can list the essential features of the heterogenous catalysis of gas-phase reactions.
1. The presence of a solid material (the heterogeneous catalyst) changes the rate of an overall chemical reaction. The term “solid” is subject to some qualification, as the sulphuric acid catalyst (see Chapter 10) is a melt held in a porous solid and some other catalysts also contain mobile phases, e.g. some potassium salts in steam reforming catalysts (see Chapter 5). The overall chemical reaction concerns the gas-phase species only, but this does not preclude the involvement of the solid in the formation of intermediate species—indeed, this is essential for heterogeneous catalysis.
2. The products of the catalysed reaction can, at least in principle, be obtained from an uncatalysed reaction under the same conditions. There is, therefore, no way of using catalysis to “cheat” equilibrium. In practice, however, the uncatalysed reaction may be immeasurably slow or may give very different products.
3. Any useful catalyst must have a high productivity. Thus, we expect 1 tonne of catalyst to “make” many tonnes of product. Alternatively, to look at catalysis on the atomic scale, the catalysed reaction steps must occur many times at the reaction site on the catalyst surface before catalytic activity is lost.
4. The catalysed reaction steps take place very close to the solid surface. These steps may be between gas molecules adsorbed on the catalyst surface, or extensive reaction can take place involving the topmost atomic layers of the catalysts. The influence of the solid does not effectively extend more than an atomic diameter into the gas phase, and the direct involvement of atoms below the topmost layers is not usually possible.
1.1.2. The Role of Catalysis
The synthesis of ammonia and its subsequent oxidation to nitric oxide give illustrations of two ways in which catalysts are essential to the chemical industry.
1.1.2.1. Ammonia synthesis
Ammonia synthesis from nitrogen and hydrogen occurs by the overall reaction shown in equation (1).
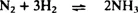 | (1) |
No reaction takes place with the reactants at ambient temperatures, and even with an active catalyst a temperature of some 400°C is needed to obtain commercially useful rates of reaction. Nothing happens in the system without a catalyst as the temperature is raised until, at temperatures higher than 1000°C, a significant proportion of the hydrogen molecules are dissociated into atoms, as shown in equation (2). For example, at 1430°C, with a pressure of molecular hydrogen of 150 bar, the partial pressure of atomic hydrogen would be 0.1% of the H2 pressure, i.e. 0.15 bar. Even this does not provide a mechanism for fixing nitrogen, for the reaction of hydrogen atoms with nitrogen molecules is very slow.2 Only above 3000°C, where the even more strongly-bound nitrogen molecules start dissociating to atoms, equation (3), does nitrogen fixation become possible.
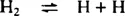 | (2) |
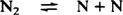 | (3) |
If each nitrogen atom formed in this way gave a molecule of ammonia by subsequent reactions with hydrogen molecules or atoms, then the rate of ammonia formation can be calculated from the rate of reaction (3).2 A 100 m3 reactor containing a 3:1 hydrogen/nitrogen mixture at a total pressure of 200 bar and a temperature of 3150°C would give 1300 tonnes of ammonia per day.
However, this simple analysis ignores the reverse reaction. Reaction (1) is an equilibrium reaction, and ammonia synthesis is favoured by low temperatures and high pressures. Thermodynamic data3 can be used to show that the partial pressure of ammonia under the conditions above cannot exceed 0.07 bar, so with a gas space velocity of 104 h−1, the make rate of ammonia would be only 6 tonnes day−1.
The role of the catalyst in ammonia synthesis is therefore that of making the reaction go sufficiently fast (by facilitating the dissociation of molecular nitrogen) so that significant rates are obtained under conditions where the equilibrium conversion is large enough to be useful (Figure 1.1).
1.1.2.2. Ammonia oxidation
The qu...
Table of contents
- Cover
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Chapter 1. Fundamental Principles
- Chapter 2. Process Design, Rating and Performance
- Chapter 3. Handling and Using Catalysts in the Plant
- Chapter 4. Feedstock Purification
- Chapter 5. Steam Reforming
- Chapter 6. The Water-gas Shift Reaction
- Chapter 7. Methanation
- Chapter 8. Ammonia Synthesis
- Chapter 9. Methanol Synthesis
- Chapter 10. Catalytic Oxidations
- Appendices
- References
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Catalyst Handbook by Martyn V. Twigg in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.