
- 111 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This book covers the history, theory, and practice of bonding elastomers to solid substrates. It provides information of methods, equipment, and bond evaluation. Numerous detailed examples of research into the variables that affect bonding, bond strength, and bond durability are provided to give the reader deeper understanding of this technology.
Trusted by 375,005 students
Access to over 1 million titles for a fair monthly price.
Study more efficiently using our study tools.
Information
1 Adhesion theory
Adhesives are essential to modern day mechanical devices. The transportation industry, both aerospace and industrial, relies on adhesives for virtually all products. Elastomers are used in many applications as flexible barriers like seals, O-rings, and diaphragms, and for energy management applications like tires, vibration isolators, and dampers (Figure 1.1). Many of these applications require that the elastomer be bonded or adhered to a substrate, commonly metal, textiles, or rigid plastic, and their ability to function would be impossible without robust rubber-to-substrate bonds. Tires, engine mounts, elastomeric bearings, vibration isolators, dampers, and the solid fuel rocket engines for the space shuttle are but a few examples.

Figure 1.1: Cross section of laminated helicopter rotor bearing (made by Lord Corporation).
Much literature has been published on the history and technology of bonding rubber to metal [1, 2, 3, 4, 5, 6, 7]. First, we will define some terms commonly used throughout the industry. ASTM D907 defines adhesion as a state in which two surfaces are held together by interfacial forces which may consist of valence forces or interlocking forces or both. The term “adhesion” refers to the strength of forces holding together two separate surfaces of the same or different materials. Thus, an adhesive is a substance that is able to hold materials together by surface attachment. “Cohesion” refers to the strength of a single material in holding itself together. In addition to having good adhesion to a substrate, an adhesive must have sufficient cohesive strength to support load. A primer is a substance applied to a substrate to generate good adhesion to the substrate, and it must also be capable of interacting with an adhesive topcoat.
Adhesion can be achieved by any combination of factors including mechanical interlocking, molecular interdiffusion, electrostatic forces, adsorption, and chemical bond formation. A number of well-known attractive forces (ionic, covalent, metallic, hydrogen, van der Waals) ensure the cohesion and adhesion of solids[8].
The forces of attraction between atoms and molecules can be divided into interatomic bonds and secondary bonds. Interatomic bonds are the strongest bonds and include ionic bonds, covalent bonds, and intermetallic bonds. Ionic bonding occurs when one of the atoms is negative (has an extra electron) and another is positive (has lost an electron). Ionic bonds are the strongest bonds, greater than 5 × 10−4 dynes/bond, and they create a strong Coulomb attraction. A good example of ionic bonding is sodium chloride salt, NaCl (Figure 1.2).
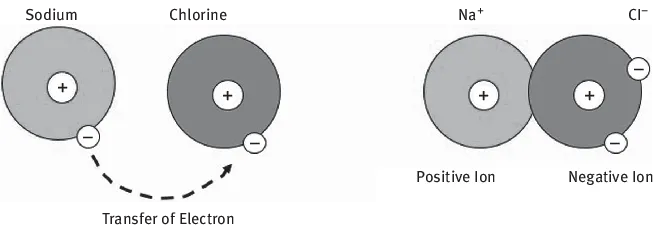
Figure 1.2: Ionic bonding.
Covalent bonding occurs when electrons are shared between molecules to saturate the valence. Covalent bonds are slightly less strong than ionic bonds, approximately 5 × 10−4 dynes/bond (Figure 1.3).
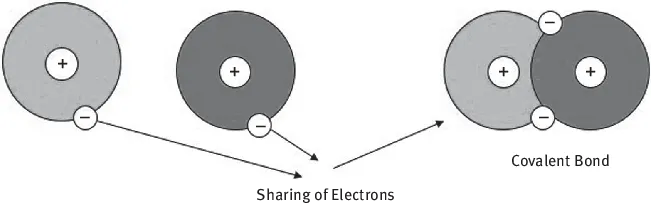
Figure 1.3: Covalent bonds.
In metals, the atoms are ionized, having lost some electrons from the valence band (the electrons are delocalized). These electrons form an electron sea which binds the charged nuclei in place. Intermetallic bonds are only about half as strong as covalent bonds, approximately 2 × 10−4 dynes/bond (Figure 1.4).
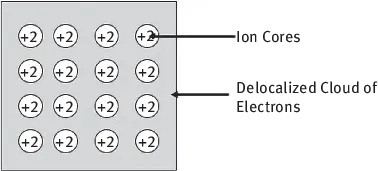
Figure 1.4: Intermetallic bonds.
Secondary bonds range from weak to very weak forces and include hydrogen bonds, London dispersion forces and Van der Waals forces. Polar molecules such as water have a weak, partial negative charge at one region of the molecule and a partial positive charge elsewhere. The positive and negative regions are attracted to the oppositely charged regions of nearby molecules. Hydrogen bonding is electrostatic because hydrogen has only a single electron in the 1s orbital and cannot form covalent bonds. Hydrogen bonding involves molecules with OH, NH or FH groups and hydrogen bonds are only 5–10% of the strength of covalent bonds, approximately 6 × 10−5 dynes/bond (Figure 1.5).
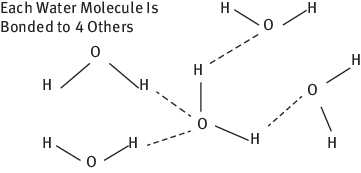
Figure 1.5: Hydrogen bonds.
Van der Waals forces, also known as dipole–dipole forces, are caused by the electrostatic attraction between polar molecules. The magnitude of force increases with the number of electrons per molecule. They are much, much weaker than chemical bonds and much weaker than hydrogen bonding, approximately 2 × 10−6 dynes/bond (Figure 1.6).
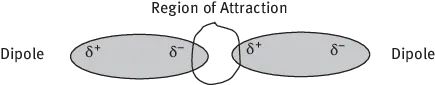
Figure 1.6: Van der Waals Forces.
London dispersion forces describe the transitory electrostatic attraction that results when the electrons of two adjacent atoms occupy positions that make the atoms form temporary dipoles. These are weak intermolecular forces that arise from the interactive forces between instantaneous dipoles in molecules without permanent dipole moments (Figure 1.7). These forces dominate the interaction of nonpolar molecules, and are significant in polar molecules. London dispersion forces are also known as “dispersion forces”, “London forces”, or “instantaneous dipole–induced dipole forces”. The strength of London dispersion forces is proportional to the polarizability of the molecule, which in turn depends on the total number of electrons and the area over which they are spread. They are approximately 2 ...
Table of contents
- Title Page
- Copyright
- Contents
- 1 Adhesion theory
- 2 History of rubber-to-metal bonding
- 3 Primers and adhesives
- 4 Rubber chemistry
- 5 Testing adhesive bonds
- 6 Substrate preparation
- 7 Adhesive application and use
- 8 Compounding effects on adhesive bonds
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Bonding of Elastomers by James R. Halladay,R J Del Vecchio in PDF and/or ePUB format, as well as other popular books in Tecnología e ingeniería & Química. We have over one million books available in our catalogue for you to explore.