
eBook - ePub
Gas Adsorption in Metal-Organic Frameworks
Fundamentals and Applications
- 530 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Gas Adsorption in Metal-Organic Frameworks
Fundamentals and Applications
About this book
This text discusses the synthesis, characterization, and application of metal-organic frameworks (MOFs) for the purpose of adsorbing gases. It provides details on the fundamentals of thermodynamics, mass transfer, and diffusion that are commonly required when evaluating MOF materials for gas separation and storage applications and includes a discussion of molecular simulation tools needed to examine gas adsorption in MOFs. Additionally, the work presents techniques that can be used to characterize MOFs after gas adsorption has occurred and provides guidance on the water stability of these materials. Lastly, applications of MOFs are considered with a discussion of how to measure the gas storage capacity of MOFs, a discussion of how to screen MOFs to for filtration applications, and a discussion of the use of MOFs to perform industrial separations, such as olefin/paraffin separations. Throughout the work, fundamental information, such as a discussion on the calculation of MOF surface area and description of adsorption phenomena in packed-beds, is balanced with a discussion of the results from research literature.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Gas Adsorption in Metal-Organic Frameworks by T. Grant Glover, Bin Mu, T. Grant Glover,Bin Mu in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
Metal–Organic Frameworks and Reticular Chemistry
T. Grant Glover
Contents
1.1Introduction
1.2Structure of Text
References
1.1Introduction
Over the last several years, metal–organic frameworks (MOFs) have captured the attention of a wide variety of research scientists. These materials are unique among porous structures because these materials provide a well-defined crystalline structure, a combination of both inorganic and organic components, a means of tailoring the functionality of the components, a wealth of structural configurations, record-breaking surface areas, and a route to understand nanopore structure and guest interactions through molecular simulations.
It is true that traditional materials, such as zeolites, structured carbons, porous polymers, and other materials, have some of these features, but typically only MOFs provide all of the features listed above. Moreover, because of the tunable nature of MOFs, thousands of MOFs have been synthesized and thousands more have been proposed in molecular simulations.1–4 It is not surprising then that the MOF field has attracted the attention of a wide variety of research scientists each searching for MOFs to solve particular technical challenges.
MOFs are a specific class of materials constructed by joining metal-containing units, termed secondary building units (SBUs), with organic linkers using strong bonds to create open crystalline frameworks with permanent porosity.4 MOF-5, shown in Figure 1.1 illustrates this concept, where the large sphere in Figure 1.1 has been added to show the volume of the adsorbent.5 With some imagination, looking at this figure illustrates the possibilities that MOFs offer. For example, the organic linker contained in the MOF can be functionalized, the length of the linker can be changed, the metal in the SBU can be varied, combinations of these variations can be completed, and many others. A small selection of MOFs and precursors are shown in Figures 1.2 and 1.3.6,7 In addition, multivariate (MTV) MOFs, where different functional groups are contained on the MOF link, and mixed-metal MOFs, where a mixture of different SBU metals are used, are shown in Figure 1.4.8
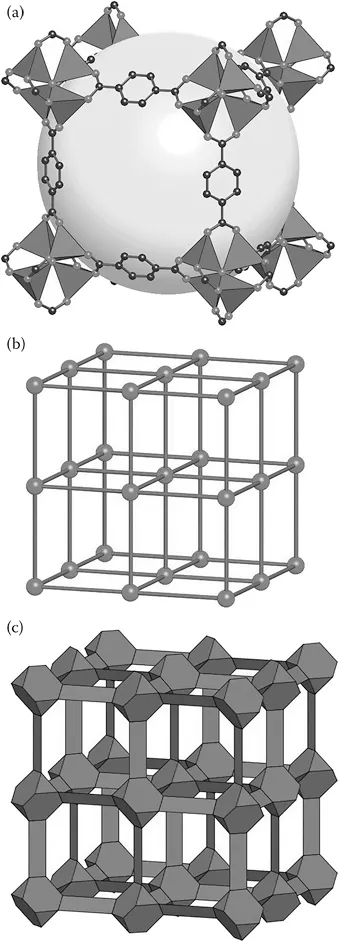
Figure 1.1(a) MOF-5, one of the first MOFs reported, illustrates the basic concept of organic links, in this case benzene dicarboxylate (O, gray spheres and C, black spheres), connected to structural building units (SBUs). The SBUs in MOF-5 are ZnO4 tetrahedra. (b) The ball and stick model of MOF-5 shows the cubic net of the material. (c) In MOF-5, the cationic clusters have a truncated tetrahedral envelope, and the rigid planar O2C–C6H4–CO2 linkers have a planar slate envelope. (Reprinted with permission from Yaghi, O. M. et al. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714.5)
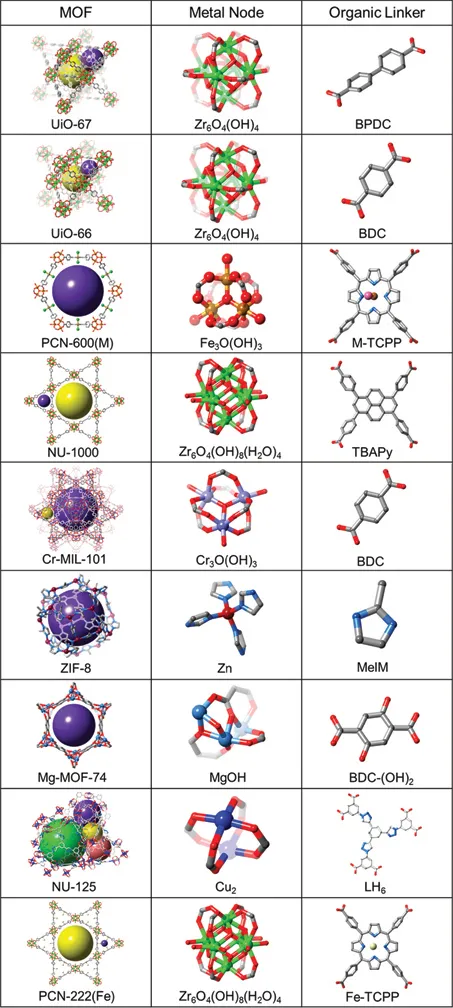
Figure 1.2Representation of MOF structures and the corresponding node and linker constituents, where Zr: green; Fe: yellow; Cr: light purple; Zn: dark red; Mg: blue; Cu: royal blue; C: grey; O: red; N: light blue; Cl: pink. (Reprinted with permission from Howarth, A. J. et al. Best Practices for the Synthesis, Activation, and Characterization of Metal–Organic Frameworks. Chem. Mater. 2017, 29, 26–39.6)
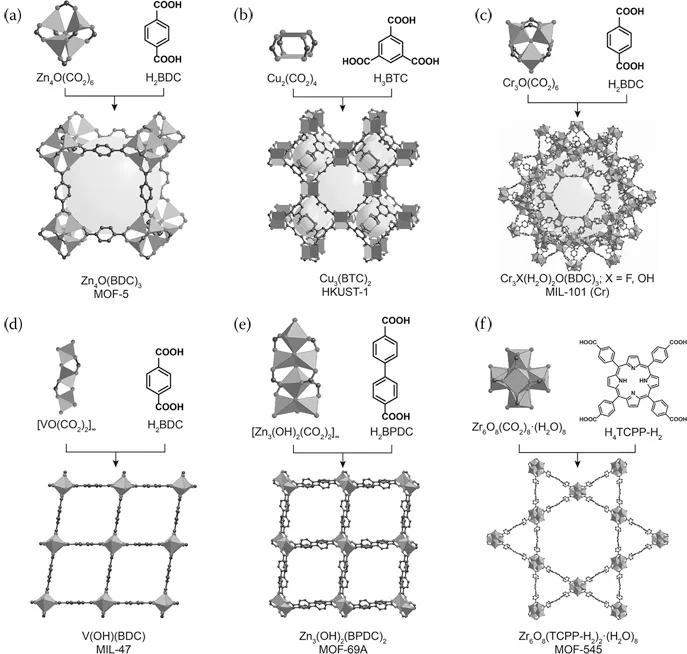
Figure 1.3Representative MOFs (a–f) showing both metal structural building units and linkers that are used to build the framework. Atom labeling scheme: C, black; O, gray; metals, gray polyhedra. H atoms are omitted for clarity. Spheres have been added to represent the space in the framework. (Reprinted with permission from Rungtaweevoranit, B. et al. Spiers Memorial Lecture: Progress and Prospects of Reticular Chemistry. Farad. Discuss. 2017, 201, 9–45.7)
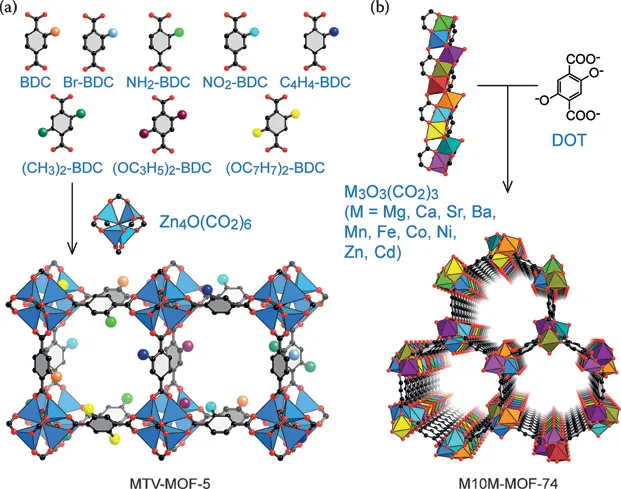
Figure 1.4(a) The multivariate (MTV) MOF concept where different links are used to construct a MOF containing multiple functional groups and (b) a mixed-metal MOF where different metals are used for structural building units. (Reprinted with permission from Furukawa, H. et al. “Heterogeneity within Order” in Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2015, 54, 3417–3430.8)
However, beyond simply synthesizing all possible MOFs, the crystalline structure of MOFs provides an opportunity to design materials on paper, or in a computer, and then synthesize a specific MOF targeted for an intended application. The process of assembling judiciously designed, molecularly-rigid, building blocks into predetermined ordered structures (networks), held together by strong bonds, is referred to as reticular synthesis.5
With the synthesis of MOFs based on well-defined building blocks, and a variety of building blocks identified, computers can be used to propose MOF structures, and the interaction of guest species in the MOF structure can be modeled using molecular simulations. The ability to utilize computational chemistry to facilitate the design of MOFs is a significant advance in the field of porous materials.
For example, the storage of natural gas on a porous material has attracted a great deal of attention in the research literature, and one MOF in particular, PCN-14, can store large amounts of natural gas.9–13 However, given the large number of MOFs that exist, and the even larger number of MOFs that can be envisioned, it is possible that other MOF structures are also suitable for natural gas storage. To examine this problem, Wilmer et al. simulated 137,953 MOFs, and predicted the methane capacity of each material.3 Over 300 MOFs were predicted with a methane storage capacity better than any known material. One material was selected for synthesis and the measured methane capacity confirmed the computationally predicted capacity.3
The concept of using computers to facilitate materials synthesis has also been illustrated hypothetically when MOFs are used as sensors. For example, a series of MOFs were selected for the construction of a sensor array and computational screening was used to predict the preferred combination of MOFs to provide the needed sensor selectivity.14 Beyond this computational stu...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Contents
- Safety and Acknowledgments
- Editors
- Contributors
- Chapter 1: Metal–Organic Frameworks and Reticular Chemistry
- Chapter 2: Synthesis and Characterization of Metal–Organic Frameworks
- Chapter 3: Thermodynamics of Adsorption
- Chapter 4: Mass Transfer in MOFs
- Chapter 5: Packed Bed Wave Theory
- Chapter 6: Simulation of Crystalline Nanoporous Materials and the Computation of Adsorption/Diffusion Properties
- Chapter 7: Characterization Techniques for the Analysis of Metal–Organic Frameworks during and after Adsorption
- Chapter 8: Water Stability of Metal–Organic Frameworks
- Chapter 9: Gas Storage in Metal–Organic Frameworks
- Chapter 10: Toxic Gas Adsorption and Reaction in Metal–Organic Frameworks
- Chapter 11: Potential Industrial Applications of Metal–Organic Frameworks for Gas Separations
- Index