
eBook - ePub
Thermodynamics Problem Solving in Physical Chemistry
Study Guide and Map
- 128 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Thermodynamics Problem Solving in Physical Chemistry: Study Guide and Map is an innovative and unique workbook that guides physical chemistry students through the decision-making process to assess a problem situation, create appropriate solutions, and gain confidence through practice solving physical chemistry problems.
The workbook includes six major sections with 20 - 30 solved problems in each section that span from easy, single objective questions to difficult, multistep analysis problems. Each section of the workbook contains key points that highlight major features of the topic to remind students of what they need to apply to solve problems in the topic area.
Key Features:
- Provides instructor access to a visual map depicting how all equations used in thermodynamics are connected and how they are derived from the three major energy laws.
- Acts as a guide in deriving the correct solution to a problem.
- Illustrates the questions students should ask themselves about the critical features of the concepts to solve problems in physical chemistry
- Can be used as a stand-alone product for review of Thermodynamics questions for major tests.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Thermodynamics Problem Solving in Physical Chemistry by Kathleen E. Murphy in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Workbook
1
Gases and Gas Laws
Key Points – Gas Laws
Ideal gas: | Van der Waals | Virial equation |
- The equation of state: applies to “ideal gases” where there are either no interactions between particles (at low T’s and P’s) or where the attractive forces between the gas particles balance the repulsive forces. The equation can be applied to all gases, independent of chemical identity.
- Real gases show deviations from ideal behavior and introduce factors that depend on the chemical identity of the gas molecule.
The van der Waals equation introduces two important factors, the values of which depend on the chemical identity of the gas:
- A correction for the molar volume the gas particles themselves occupy, represented by the term “b”, which is subtracted from the total molar volume, appears in the first term of the equation.
- The second factor, “a”, is a measure of the attractive forces between the gas molecules, represented by the term “an2/V2 or a/Vm2”, which is subtracted from the adjusted first term.
- The a and b values are tabled for each gas as van der Waals constants.
The virial equation describes gas isotherms as polynomials in V or Vm, where the second virial coefficient B, can be related to “a” and “b” from the van der Waals equation. The value of B is very temperature dependent, different for each gas and may be positive or negative. The third factor, “C”, in the equation is rarely needed to define the behavior of a gas.
- The compressibility factor, Z, is a useful parameter with which to determine when a gas is NOT acting ideally, since
Z < 1.0 The gas is not acting ideally and attractive forces dominate. Vm,obs < Videal Z = 1.0 The gas is acting ideally Vm,obs = Videal Z > 1.0 The gas is not acting ideally and repulsive forces dominate. Vm,obs > Videal | 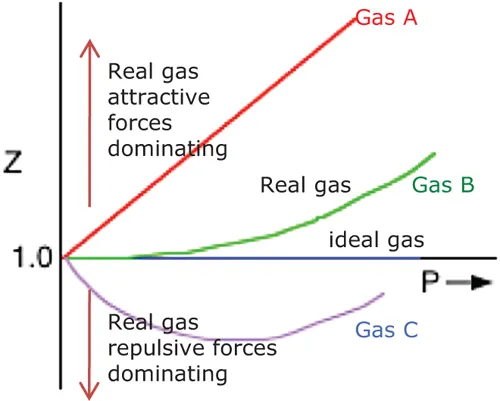 |
The values of Z vary with T and can be determined by comparing observed values of P, V or other gas properties with those predicted by the ideal gas law.
The ideal gas law allows for the determination of molecular weights or molar masses of gaseous species, as well as the density of a gas at any P and T combination, if the chemical identity o...
Table of contents
- Cover
- Half-Title
- Title
- Copyright
- Contents
- Preface
- Author
- Workbook
- Final Answers
- Index