
- 524 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
A Q&A Approach to Organic Chemistry
About this book
A Q&A Approach to Organic Chemistry is a book of leading questions that begins with atomic orbitals and bonding. All critical topics are covered, including bonding, nomenclature, stereochemistry, conformations, acids and bases, oxidations, reductions, substitution, elimination, acyl addition, acyl substitution, enolate anion reactions, the Diels–Alder reaction and sigmatropic rearrangements, aromatic chemistry, spectroscopy, amino acids and proteins, and carbohydrates and nucleosides. All major reactions are covered. Each chapter includes end-of-chapter homework questions with the answer keys in an Appendix at the end of the book. This book is envisioned to be a supplementary guide to be used with virtually any available undergraduate organic chemistry textbook. This book allows for a "self-guided" approach that is useful as one studies for a coursework exam or as one reviews organic chemistry for postgraduate exams.
Key Features:
- Allows a "self-guided tour" of organic chemistry
- Discusses all important areas and fundamental reactions of organic chemistry
- Classroom tested
- Useful as a study guide that will supplement most organic chemistry textbooks
- Assists one in study for coursework exams or allows one to review organic chemistry for postgraduate exams
- Includes 21 chapters of leading questions that covers all major topics and major reactions of organic chemistry
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Part A
A Q&A Approach to Organic Chemistry
1
Orbitals and Bonding
1.1 ORBITALS
1.1.1 Atomic Orbitals
- What is the electronic structure of an atom?
- What is the Schrödinger wave equation?
- What are atomic orbitals?
- What is a node?
- What is the Heisenberg uncertainty principle?
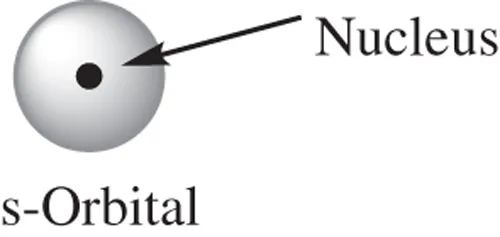
- What is a s-orbital?
- What is a p-orbital?
Table of contents
- Cover
- Half-Title
- Title
- Copyright
- Contents
- Preface
- Common Abbreviations
- Author
- A Q&A Approach to Organic Chemistry
- A Q&A Approach to Organic Chemistry
- Appendix: Answers to End of Chapter Problems
- Index