
- 562 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handbook of Petrochemical Processes
About this book
The petrochemical industry is a scientific and engineering field that encompasses the production of a wide range of chemicals and polymers. The purpose of this book is not only to provide a follow-on to form the later chapters of the highly successful Chemistry and Technology of Petroleum 5th Edition but also provides a simplified approach to a very diverse chemical subject dealing with the chemistry and technology of various petroleum and petrochemical process. Following from the introductory chapters, this book provides the readers with a valuable source of information containing insights into petrochemical reactions and products, process technology, and polymer synthesis.
- Provides readers with a valuable source of information containing insights into petrochemical reactions and products, process technology, and polymer synthesis
- Introduces the reader to the various petrochemical intermediates are generally produced by chemical conversion of primary petrochemicals to form more complicated derivative products
- The reactions and processes involved in transforming petroleum-based hydrocarbons into the chemicals that form the basis of the multi-billion dollar petrochemical industry are reviewed and described
- The book includes information on new process developments for the production of raw materials and intermediates for petrochemicals
- Includes a description of the origin of the raw materials for the petrochemicals industry – including an overview of the coal chemicals industry
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Handbook of Petrochemical Processes by James G. Speight in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1 The Petrochemical Industry
1.1 INTRODUCTION
The constant demand for hydrocarbon products such as liquid fuels is one of the major driving forces behind the petroleum industry. However, the other driving force is a major group of hydrocarbon products (petrochemicals) that are the basis of a major industry. There is a myriad of products that have evolved through the short life of the petroleum industry, either as bulk fractions or as single hydrocarbon products (Tables 1.1 and 1.2). And the complexities of product composition have matched the evolution of the products. In fact, it is the complexity of product composition that has served the industry well and, at the same time, had an adverse effect on product use.
A petrochemical is a chemical product developed from petroleum that has become an essential part of the modern chemical industry (Table 1.3) (Speight, 1987; Parkash, 2003; Gary et al., 2007; Speight, 2014; Hsu and Robinson, 2017; Speight, 2017). The chemical industry is, in fact, the chemical process industry by which a variety of chemicals are manufactured. The chemical process industry is, in fact, subdivided into other categories that are: (i) the chemicals and allied product industries in which chemicals are manufactured from a variety of feedstocks and may then be put to further use, (ii) the rubber and miscellaneous product industries which focus on the manufacture of rubber and plastic materials, and (iii) petroleum refining and related industries which, on the basis of the following chapters in this text, is now self-explanatory. Thus, the petrochemical industry falls under the subcategory of petroleum and related industries.
In the context of this book, the definition of petrochemicals excludes fuel products, lubricants, asphalt, and petroleum coke but does include chemicals produced from other feedstocks such as coal, oil shale, and biomass, which could well be the sources of chemicals in the future. Thus, petrochemicals are, in the strictest sense, different to petroleum products insofar, as the petrochemicals are the basic building blocks of the chemical industry. Petrochemicals are found in products as diverse as plastics, polymers, synthetic rubber, synthetic fibers, detergents, industrial chemicals, and fertilizers (Table 1.3). Petrochemicals are used for production of several feedstocks and monomers and monomer precursors. The monomers after polymerization process create several polymers, which ultimately are used to produce gels, lubricants, elastomers, plastics, and fibers.
By way of definition and clarification as it applies to the petrochemical and chemical industry, primary raw materials are naturally occurring substances that have not been subjected to chemical changes after being recovered. Currently, through a variety of intermediates petroleum and natural gas are the main sources of the raw materials because they are the least expensive, most readily available, and can be processed most easily into the primary petrochemicals. An aromatic petrochemical is also an organic chemical compound but one that contains, or is derived from, the basic benzene ring system.
TABLE 1.1
The Various Distillation Fractions of Petroleum
The Various Distillation Fractions of Petroleum

TABLE 1.2
Properties of Hydrocarbon Products from Petroleum
Properties of Hydrocarbon Products from Petroleum

Primary petrochemicals include: (i) olefin derivatives such as ethylene, propylene, and butadiene; (ii) aromatic derivatives such as benzene, toluene, and the isomers of xylene (BTX); and (iii) methanol. However, although petroleum contains different types of hydrocarbon derivatives, not all hydrocarbon derivatives are used in producing petrochemicals. Petrochemical analysis has made it possible to identify some major hydrocarbon derivatives used in producing petrochemicals (Speight, 2015). From the multitude of hydrocarbon derivatives, those hydrocarbon derivatives serving as major raw materials used by petrochemical industries in the production of petrochemicals are: (i) the raw materials obtained from natural gas processing such as methane, ethane, propane, and butane; (ii) the raw materials obtained from petroleum refineries such as naphtha and gas oil; and (iii) the raw materials such as benzene, toluene and the xylene isomers obtained when extracted from reformate (the product of reforming processes through catalysts called catalytic reformers in petroleum refineries (Parkash, 2003; Gary et al., 2007; Speight, 2008, 2014; Hsu and Robinson, 2017; Speight, 2017).
TABLE 1.3
Examples of Products from the Petrochemical Industry
Examples of Products from the Petrochemical Industry

Thus, petrochemicals are chemicals derived from petroleum and natural gas and, for convenience of identification, petrochemicals can be divided into two groups: (i) primary petrochemicals and (ii) intermediates and derivatives (Figure 1.1).
Primary petrochemicals include: olefins (ethylene, propylene, and butadiene), aromatics (benzene, toluene, and xylenes), and methanol. Petrochemical intermediates are generally produced by chemical conversion of primary petrochemicals to form more complicated derivative products. Petrochemical derivatives can be made in a variety of ways: (i) directly from primary petrochemicals; (ii) through intermediate products which still contain only carbon and hydrogen; and (iii) through intermediates which incorporate chlorine, nitrogen, or oxygen in the finished derivative. In some cases, they are finished products; in others, more steps are needed to be arrived at the desired composition.
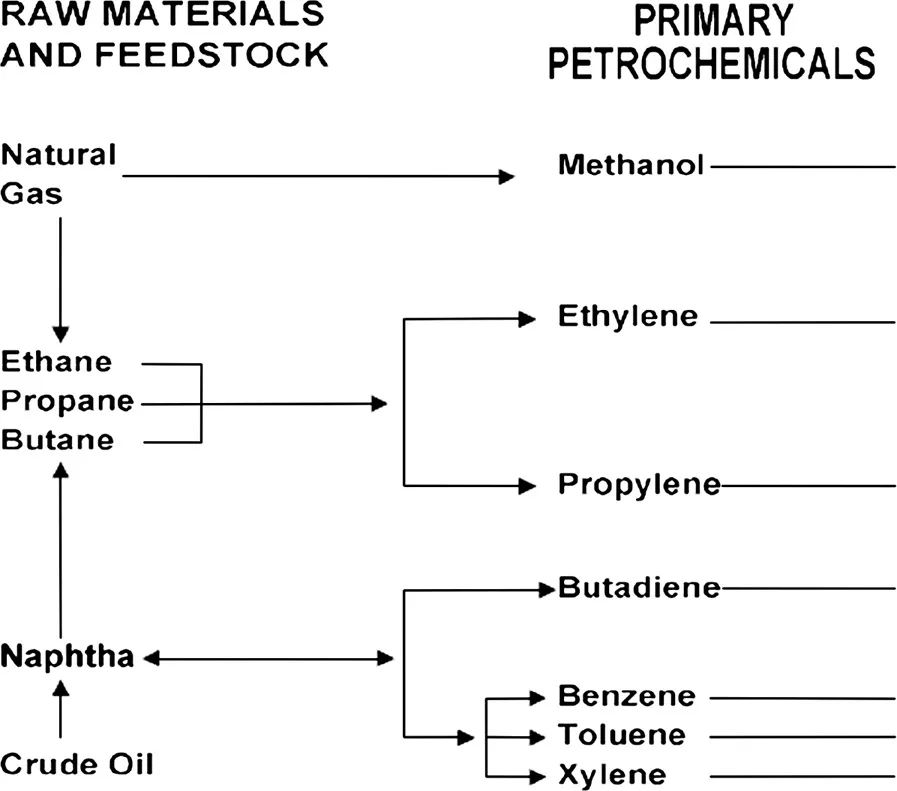
FIGURE 1.1 Raw materials and primary petrochemicals. (Speight, J.G. 2007. The Chemistry and Technology of Petroleum. 4th Edition. CRC Press, Boca Raton, FL. Figure 27.3, p. 784.)
Moreover, petrochemical feedstocks can be classified into several general groups: olefins, aromatics, and methanol; a fourth group includes inorganic compounds and synthesis gas (mixtures of carbon monoxide and hydrogen). In many instances, a specific chemical included among the petrochemicals may also be obtained from other sources, such as coal, coke, or vegetable products. For example, materials such as benzene and naphthalene can be made from either petroleum or coal, while ethyl alcohol may be of petrochemical or vegetable origin.
Thus, primary petrochemicals are not end products, but are the chemical building blocks for a wide range of chemical and manufactured materials. For example, petrochemical intermediates are generally produced by chemical conversion of primary petrochemicals to form more complicated derivative products (Parkash, 2003; Gary et al., 2007; Speight, 2008, 2014; Hsu and Robinson, 2017; Speight, 2017). Petrochemical derivative products can be made in a variety of ways: (i) directly from primary petrochemicals; (ii) through intermediate products which still contain only carbon and hydrogen; and (iii) through intermediates which incorporate chlorine, nitrogen, or oxygen in the finished derivative. In some cases, they are finished products; in others, more steps are needed to arrive at the desired composition. Some typical petrochemical intermediates are: (i) vinyl acetate (CH3CO2CH=CH2) for paint, paper, and textile coatings; (ii) vinyl chloride (CH2=CHCl) for polyvinyl chloride (PVC); (iii) ethylene glycol (HOCH2CH2OH) for polyester textile fibers; and (iv) styrene (C6H5CH=CH2), which is important in rubber and plastic manufacturing. Of all the processes used, one of the most important is polymerization (Chapter 11). It is used in the production of plastics, fibers, and synthetic rubber, the main finished petrochemical derivatives.
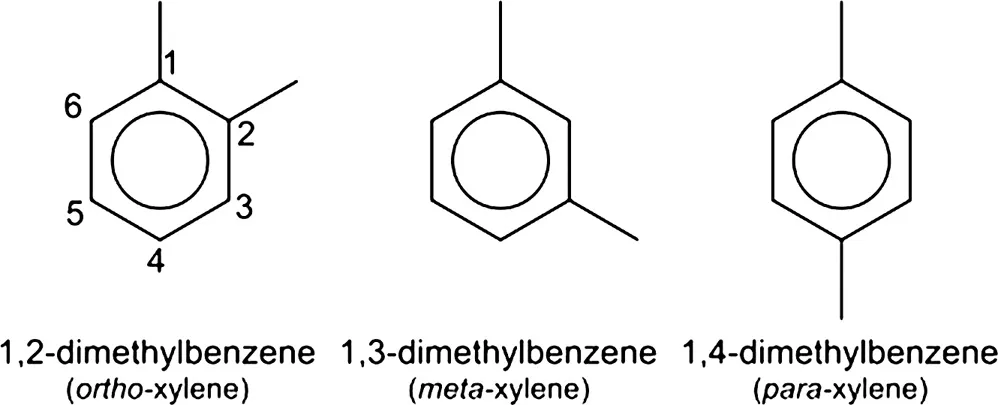
Following from this, secondary raw materials, or intermediate chemicals (Chapters 5 and 6), are obtained from a primary raw material through a variety of different processing schemes. The intermediate chemicals may be low-boiling hydrocarbon compounds such as methane, ethane, propane, and butane or higher-boiling hydrocarbon mixtures such as naphtha, kerosene, or gas oil. In the latter cases (naphtha, kerosene, and gas oil), these fractions are used (in addition to the production of fuels) as feedstocks for cracking processes to produce a variety of petrochemical products (e.g., ethylene, propylene, benzene, toluene, and the xylene isomers), which are identified by the relative placement of the two methyl groups on the aromatic ring:
Also, by way of definition, petrochemistry is a branch of chemistry in which the transformation of petroleum (crude oil) and natural gas into useful products or feedstock for other process is studied. A petrochemical plant is a plant that uses chemicals from petroleum as a raw material (the feedstock) are usually located adjacent to (or within the precinct of) a petroleum refinery in order to minimize the need for transportation of the feedstocks produced by the refinery (Figure 1.2). On the other hand, specialty chemical plants and fine chemical plants are usually much smaller than a petrochemical plant and are not as sensitive to location.
Furthermore, a paraffinic petrochemical is an organic chemical compound, but one that does not contain any ring systems such as a cycloalkane (naphthene) ring or an aromatic ring. A naphthenic petrochemical is an organic chemical compound that contains one or more cycloalkane ring systems. An aromatic petrochemical is also an organic chemical compound but one that contains, or is derived from, the basic benzene ring system.

FIGURE 1.2 Schematic diagram of a refinery showing the production of products during the distillation and during thermal processing (e.g., visbreaking, coking, and catalytic cracking).
Petroleum products (in contrast to petrochemicals) are those hydrocarbon fractions that are derived from petroleum and have commercial value as a bulk product (Tables 1.1 and 1.2) (Parkash, 2003; Gary et al., 2007; Speight, 2014; Hsu and Robinson, 2017; Speight, 2017). These products are generally not accounted for in petrochemical production or used in statistics. Thus, in the context of this definition of petrochemicals, this book focuses on chemicals that are produced from petroleum as distinct from petroleum products, which are organic compounds (typically hydrocarbon compounds) that are burned as a fuel. In the strictest sense of the definition, a petrochemical is any chemical that is manufactured from petroleum and natural gas as distinct from fuels and other products, which are derived from petroleum and natural gas by a variety of processes and used for a variety of commercial purposes (Chenier, 2002; Meyers 2005; Naderpour, 2008; Speight, 2014). Petrochemical products include such items as plastics, soaps and detergents, solvents, drugs, fertilizers, pesticides, explosives, synthetic fibers and rubbers, paints, epoxy resins, and flooring and insulating materials.
Moreover, the classification of materials as petrochemicals is used to indicate the source of the chemical compounds, but it should be remembered that many common petrochemicals can be made from other sources, and the terminology is therefore a matter of source identification. However, in the setting of modern industry, the term petrochemicals, is often used in an expanded form to include chemicals produced from other fossil fuels such as coal or natural gas, oil shale, and renewable sources such as corn or sugar cane as well as other forms of biomass (Chapter 3). It is in the expanded form of the definition that the term petrochemical is used in this book.
In fact, in the early days of the chemical industry, coal was the major source of chemicals (it was not then called the petrochemical industry) and it was only after the discovery of petroleum and the recognition that petroleum could produce a variety of products other than fuels that the petrochemical industry came into being (Spitz, 1988; Speight, 2013, 2014, 2017). For several decades both coal and petroleum served as the primary raw materials for the manufacture of chemicals. Then during the time of World War II, petroleum began to outpace coal as a source of chemicals—the exception being the manufacture of synthetic fuels from coal because of the lack of access to petroleum by German industry.
To complete this series of definitions and to reduce the potential for any confusion that might occur later in this text, specialty chemicals (also called specialties or effect chemicals) are particular chemical products, which provide a wide variety of effects on which many other industry sectors rely. Specialty chemicals are materials used on the basis of their performance or function. Consequently, in addition to effect chemicals they are sometimes referred to as performance chemicals or formulation chemicals. The physical and chemical characteristics of the single molecules or the formulated mixtures of molecules and the composition of the mixtures influence the performance of the end product.
On the other hand, the term fine chemicals is used in distinction to heavy chemicals, which are produced and handled in large lots and are often in a crude state. ...
Table of contents
- Cover
- Half Title
- Series Page
- Title Page
- Copyright Page
- Table of Contents
- Preface
- About the Author
- Chapter 1 The Petrochemical Industry
- References
- Chapter 2 Feedstock Composition and Properties
- References
- Chapter 3 Other Feedstocks—Coal, Oil Shale, and Biomass
- References
- Chapter 4 Feedstock Preparation
- References
- Chapter 5 Feedstock Preparation by Gasification
- References
- Chapter 6 Chemicals from Paraffin Hydrocarbons
- References
- Chapter 7 Chemicals from Olefin Hydrocarbons
- References
- Chapter 8 Chemicals from Aromatic Hydrocarbons
- References
- Chapter 9 Chemicals from Non-hydrocarbons
- References
- Chapter 10 Chemicals from the Fischer–Tropsch Process
- References
- Chapter 11 Monomers, Polymers, and Plastics
- References
- Chapter 12 Pharmaceuticals
- References
- Conversion Tables
- Glossary
- Index