
- 542 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Battery Technology Handbook
About this book
This practical reference remains the most comprehensive guide to the fundamental theories, techniques, and strategies used for battery operation and design. It includes new and revised chapters focusing on the safety, performance, quality, and enhancement of various batteries and battery systems. From automotive, electrochemical, and high-energy applications to system implementation, selection, and standardization, the Second Edition presents expert discussions on electrochemical energy storage, the advantages of battery-powered traction, the disposal and recycling of used batteries, hazard prevention, and the chemistry and physics of lithium primary batteries.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
eBook ISBN
97811355276791
Electrochemical Energy Storage
1.1 INTRODUCTION
Electrical energy plays an important role in our daily life. It can universally be applied and easily be converted into light, heat or mechanical energy. A general problem, however, is that electrical energy can hardly be stored. Capacitors allow its direct storage, but the quantities are small, compared to the demand of most applications. In general, the storage of electrical energy requires its conversion into another form of energy. In batteries the energy of chemical compounds acts as storage medium, and during discharge, a chemical process occurs that generates energy which can be drawn from the battery in form of an electric current at a certain voltage.
For a number of battery systems this process can be reversed and the battery recharged, i.e. the intake of electric energy can restore the chemical composition that contains higher energy and can closely reestablish the original structures within the battery.
As a consequence, two different battery systems exist:
● Primary batteries that are designed to convert their chemical energy into electrical energy only once.
● Secondary batteries that are reversible energy converters and designed for repeated discharges and charges. They are genuine electrochemical storage systems.
There is no clear border between them, and some primary battery systems permit charging under certain conditions. Usually, however, their rechargeability is limited.
The first part of this book (Chapters 2 to 14) concerns batteries of larger capacities that are employed as standby batteries in stationary applications, provide energy in vehicles like forklift trucks, or stabilize an electrical network like the starter battery in motor cars. Rechargeable batteries usually are the choice in such applications, since primary batteries would be too expensive for the required rather high capacity. The second part (Chapters 15 to 19) regards batteries mainly in portable applications and concerns smaller capacities. In this field primary as well as secondary batteries are employed.
1.2 THE ELECTROCHEMICAL CELL AND THE CELL REACTION
The cell reaction is a chemical reaction that characterizes the battery. When the battery is discharged, chemical compounds of higher energy content are converted by this reaction into compounds of lower energy content. Usually the released energy would be observed as heat. But in a battery, the cell reaction is divided into two electrode reactions, one that releases electrons and the other one that absorbs electrons, and this flow of electrons forms the current that can be drawn from the battery. Thus the generation or consumption of energy that is connected to the cell reaction is directly converted into an electric current. This is achieved in the electrochemical cell, sketched in Fig. 1.1.
A positive and a negative electrode are immersed in the electrolyte and the reacting substances (the active material) usually are stored within the electrodes, sometimes also in the electrolyte, if it participates in the overall reaction. During discharge, as shown in Fig. 1.1, the negative electrode contains the substance that is oxidized (i.e. releases electrons), while the positive electrode contains the oxidizing substance that is reduced (i.e. accepts electrons).
Thus at the negative electrode oxidation of S(N)red occurs according to
S(N)red ⇒ S(N)ox, + n ·e–(1a)
while S(P)ox is reduced at the positive electrode
S(P)ox + n · e– ⇒ S(P)red(1b)
Both together form the cell reaction
S(N)red + S(P)ox ⇒ S(N)ox + S(P)red + energy(1)
When the battery belongs to the secondary type and is charged, this reaction is reversed and a corresponding amount of energy has to be supplied to the cell.
The difference of the bonding energy between the composition at the starting point of the cell reaction (S(N)red+S(P)ox) and its final state (S(N)ox+S(P)red) represents the energy that can be drawn from the cell as a current (except the reversible heat (Section 1.4.1) that is lost as heat or gained as additional energy and except other losses that produce Joule heating (Section 1.4.2)). This direct conversion of the current into chemical energy characterizes batteries and fuel cells. Other systems, like combustion engines, use also a chemical reaction where a ‘fuel’ is oxidized, but in these devices the energy is generated as heat and has to be converted by further processes into mechanical or electrical energy. The advantage of the direct energy conversion is its high efficiency.
Examples of such cell reactions are
Zn + 2MnO2 ⇒ ZnO + Mn2O3(2)
for a primary battery (Leclanché battery), where zinc (Zn) and manganese dioxide (MnO2) are the compounds of higher energy content and
Cd + 2Ni(OOH) + H2O ⇒ 2Ni(OH)2Cd(OH)2(3)
as the (simplified) cell reaction of the rechargeable nickel/cadmium battery. In this case cadmium (Cd) and nickel hydroxide (Ni(OOH)), which contains Ni3+ ions, are the reactants of higher energy content.
Mostly in batteries the reacting substances are stored within the electrodes (the ‘active material’), but there are also systems where the electrolyte participates, as in lead-acid batteries, or where the reacting substances are stored in separate tanks, e.g. Zn/Cl, Zn/Br, and vanadium redox batteries (Section 1.8.5), or as a gas in the container of nickel-hydrogen batteries (Section 1.8.3).
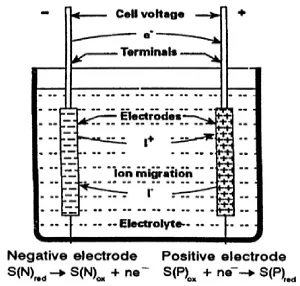
Figure 1.1 The electrochemical cell and the split up of the cell reaction. S(N)red and S(P)ox are the components of the negative and the positive electrode respectively. They are oxidized into S(N)ox at the negative and reduced into S(P)red at the positive electrode, when the battery is discharged as indicated in the figure.
According to the definition of the terms ‘anodic’ and ‘cathodic’, given in Section 1.5.1, in the situation shown, the positive electrode is the ‘cathode’ and the negative electrode the ‘anode’.
Fuel cells are also based on an electrochemical cell as shown in Fig. 1.1, but in fuel cells the reacting substances are supplied from outside, and the electrodes only provide the surface for the reaction and the connection to current flow. For this reason, fuel cells do not store electric energy, but are converters of energy, and storage parameters, like Wh/kg or Wh/L, have no relevance for them. Therefore, fuel cells cannot directly be compared with batteries.
Note: The arrangement shown in Fig. 1.1 resembles an electrolytic capacitor where also two electr...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Preface to the Second Edition
- Preface to the First Edition
- Table of Contents
- Contributors
- I. Fundamentals and Theory, Running Techniques, Applications, and Outlook: Traction Batteries, Stationary Batteries, and Charging Methods
- II. Portable Batteries
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Battery Technology Handbook by H.A. Kiehne in PDF and/or ePUB format, as well as other popular books in Tecnologia e ingegneria & Ingegneria elettronica e telecomunicazioni. We have over one million books available in our catalogue for you to explore.