
- 664 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Environmental Engineer's Mathematics Handbook
About this book
Advanced mathematics used in engineering is studied here in this text which examines the relationship between the principles in natural processes and those employed in engineered processes. The text covers principles, practices and the mathematics involved in the design and operation of environmental engineering works. It also presents engineering
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
PART I
Fundamental Computation and Modeling
CHAPTER 1
Conversion Factors and SI Units
1.1 INTRODUCTION
The units most commonly used by environmental engineering professionals are based on the complicated English System of Weights and Measures. However, bench work is usually based on the metric system or the International System of Units (SI) because of the convenient relationship among milliliters (mL), cubic centimeters (cm3), and grams (g).
The SI is a modernized version of the metric system established by international agreement. The metric system of measurement was developed during the French Revolution and was first promoted in the U.S. in 1866. In 1902, proposed congressional legislation requiring the U.S. government to use the metric system exclusively was defeated by a single vote. Although we use both systems in this text, SI provides a logical and interconnected framework for all measurements in engineering, science, industry, and commerce. The metric system is much simpler to use than the existing English system because all its units of measurement are divisible by 10.
Before we list the various conversion factors commonly used in environmental engineering, we describe the prefixes commonly used in the SI system. These prefixes are based on the power 10. For example, a “kilo” means 1000g, and a “centimeter” means 1/100 of 1m. The 20SI prefixes used to form decimal multiples and submultiples of SI units are given in Table 1.1.
Note that the kilogram is the only SI unit with a prefix as part of its name and symbol. Because multiple prefixes are not used, in the case of the kilogram the prefix names of Table 1.1 are used with the unit name “gram” and the prefix symbols are used with the unit symbol “g.” With this exception, any SI prefix may be used with any SI unit, including the degree Celsius and its symbol °C.
Example 1.1
10−6kg=1mg (1 milligram), but not 10−6kg=1μkg (1 microkilogram)
Example 1.2
Consider the height of the Washington Monument. We may write hw=169,000mm=16,900cm= 169m=0.169km, using the millimeter (SI prefix “milli,” symbol “m”); centimeter (SI prefix “centi,” symbol “c”); or kilometer (SI prefix “kilo,” symbol “k”).
1.2 CONVERSION FACTORS
Conversion factors are given in alphabetical order in Table 1.2 and in unit category listing order in Table 1.3.
Table 1.1 SI Prefixes
Table 1.2 Alphabetical Listing of Conversion Factors
Table 1.3 Conversion Factors by Unit Category
Example 1.3
Problem:
Find degrees in Celsius of water at 72°F.
Solution:
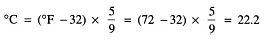
1.3 CONVERSION FACTORS: PRACTICAL EXAMPLES
Sometimes we must convert between different units. Suppose that a 60-in. piece of pipe is attached to an existing 6-ft piece of pipe. Joined together, how long are they? Obviously, we cannot find the answer to this question by adding 60 to 6. Why?—because the two lengths are given in different units. Before we can add the two lengths, we must convert one of them to the units of the other. Then, when we have two lengths in the same units, we can add them.
To perform this conversion, we need a conversion factor. In this case, we need to know how many inches make up a foot—that is, 12in. is 1ft. Knowing this, we can perform the calculation in two steps:
- 60in. is really 60/12=5ft
- 5ft+6ft=11ft
From this example, we see that a conversion factor changes known quantities in one unit of measure to an equivalent quantity in another unit of measure.
In making the conversion from one unit to another, we must know two things:
- The exact number that relates the two units
- Whether to multiply or divide by that number
When conversions are necessary, confusion over whether to multiply or divide is common; however, the number that relates the two units is usually known and thus is not a problem. Understanding the proper methodology—the “mechanics”—to use for various operations requires practice and common sense.
Along with using the proper mechanics (and practice and common sense) in making conversions, probably the easiest and fastest method of converting units is to use a conversion table. The simplest conversions require that the measurement be multiplied or divided by a constant value. For instance, if the depth of wet cement in a form is 0.85ft, multiplying by 12in./ft converts the measured depth to inches (10.2in.). Likewise, if the depth of the cement in the form is measured as 16in., dividing by 12in./ft converts the depth measurement to feet (1.33ft).
1.3.1 Weight, Concentration, and Flow
Using Table 1.4 to convert from one unit expression to another and vice versa is good practice. However, in making conversions to solve process computations in water treatment operations, for example, we must be familiar with conversion calculations based upon a relationship among weight, flow or volume, and concentration. The basic relationship is
Weight=Concentration×Flow or Volume×Factor
(1.1)
(1.1)
Table 1.5 summarizes weight, volume, and concentration calculations. With practice, many of these calculations become second nature to users.
Table 1.4 Conversion Table
Table 1.5 Weight, Volume, and Concentration Calculations
The following conversion factors are used extensively in environmental engineering (water and wastewater operations):
- 7.48gal/ft3
- 3.785L/gal
- 454g/lb
- 1000mL/L
- 1000mg/g
- 1ft3/sec (cfs)=0.6465MGD (million gallons per day)
Key point: Density (also called specific weight) is mass per unit volume and may be registered as pounds per cubic foot; pounds per gallon; grams per milliliter; or grams per cubic meter. If we take a fixed volume container, fill it with a fluid, and weigh it, we can determine density of the fluid (after subtracting the weight of the container).
- 8.34lb/gal (water)—(density=8.34lb/gal)
- 1mL of water weighs 1g—(density=1g/mL)
- 62.4lb/ft3 (water)—(density=8.34lb/gal)
- 8.34lb/gal=milligrams per liter (converts dosage in milligrams per liter into pounds per day per million gallons per day)
Example: 1mg/L×10MGD×8.3=83.4lb/day
- 1psi=2.31ft of water (head)
- 1 foot head=0.433psi
- °F=9/5(°C+32)
- °C=5/9(°F−32)
- Average water usage: 100 gal/capita/day (gpcd)
- Persons per single-family residence: 3.7
1.3.2 Water/Wastewater Conversion Examples
Use Table 1.4 and Table 1.5 to make the conversions indicated in the following example problems. Other conversions are presen...
Table of contents
- Cover Page
- Title Page
- Copyright Page
- Preface
- Acknowledgments
- Part I: Fundamental Computation and Modeling
- Part II: Fundamental Science and Statistics Review
- Part III: Math Concepts: Air Pollution Control
- Part IV: Math Concepts: Water Quality
- Part V: Math Concepts: Wastewater Engineering
- Part VI: Math Concepts: Stormwater Engineering
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Environmental Engineer's Mathematics Handbook by Frank R. Spellman,Nancy E. Whiting in PDF and/or ePUB format, as well as other popular books in Mathematics & Probability & Statistics. We have over one million books available in our catalogue for you to explore.