
- 234 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Concise Chemical Thermodynamics
About this book
The first two editions of Concise Chemical Thermodynamics proved to be a very popular introduction to a subject many undergraduate students perceive to be difficult due to the underlying mathematics. With its concise explanations and clear examples, the text has for the past 40 years clarified for countless students one of the most complicated bran
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 Energy
1.1 THE REALM OF THERMODYNAMICS
Our world is characterized by a multitude of natural phenomena. It is a world of change, of movement and energy–of storms, earthquakes, cosmic rays, and solar flares. The range and complexity of these changes is so great that it would seem the height of foolishness to attempt to find a common thread or theme that runs through all of them. Nonetheless, over the centuries, by patient and careful observation aided by the occasional flash of insight, men have come to a partial understanding of the factors involved. At the heart of such a science is the concept of energy. Thermodynamics, as we now know it, derived its name originally from studies of the “motive power of heat” and had to do primarily with steam engines and their efficient use. The main reason why the idea of energy was so poorly understood is that energy, the capacity for doing work, appears in so many different forms. However, after heat and work were understood to be but different forms of the same thing and calculations of the efficiencies of steam engines were carried out, thermodynamics was applied more generally to all changes, both chemical and physical.
Thermodynamics is a science of the macroscopic world. That is, it requires no prior understanding of atomic and molecular structure, and all its measurements are made on materials en masse. This is not to say that an understanding of molecular phenomena cannot help us to grasp some difficult concepts. The branch of the subject known as statistical thermodynamics has assisted greatly in our understanding of entropy, for example, but the basic theories of thermodynamics are formulated quite independently of it. This point is even more evident if we consider a complete description of, for example, the steam in a kettle of boiling water. A description in molecular terms would involve the position and nature of each particle, and its velocity at any instant. As there would be well over 1021 molecules present, this would be a humanly impossible task. On the macroscopic scale, however, we are glad to find that the chemical composition of steam, its temperature and pressure, for example, are quite sufficient to specify the situation. If we accept that such variables as temperature, pressure, and composition are a sufficient description of such a system, and if we are prepared to follow energy in all its various disguises, then we shall find that thermodynamics is a reliable pathfinder and guide to new and unexplored phenomena. We shall find that by taking relatively simple measurements such as heats of reaction and specific heats, we can predict the outcome, and even calculate the equilibrium constants, of changes that may never have been attempted before.
Thermodynamics is a reliable guide in industrial chemistry, plasma physics, space technology, and nuclear engineering, to name but a few applications. We shall now discuss some aspects of the two main fields of application, which spring from the first and second laws of thermodynamics. The first law is the energy conservation law, which requires a clear understanding of energy’s disguises. The second law deals with the concept of entropy, an increase of which may be regarded as one of nature’s two fundamental forces. (The other driving force is the minimization of energy.)
1.1.1 ENERGY BOOKKEEPING
The rate of physical and mental development of the species Homo sapiens was previously limited by naturally occurring genetic processes. Revolutionary extensions to man’s faculties have been made in the last two centuries. They are the extension of his mental capacity that came with the introduction of the electronic computer in the mid-twentieth century, and the extension of his physique that came with the development of machinery in the Industrial Revolution. We shall be concerned with this second aspect. There is a close link between the per capita consumption of energy and the state of physical advancement of a nation.
The current (2009) economic downturn is dampening near-term world energy demand growth. In the International Energy Outlook 2009 (IEO2009) projections from the U.S. Energy Information Administration (EIA), total world consumption of marketed energy is projected to grow by 44% between 2006 and 2030 as economic recovery spurs future demand growth (Figure 1.1 and Table 1.1) [1].
The largest projected increase in energy demand is for the non-OECD economies. The OECD (Organization for Economic Cooperation and Development) groups 30 member countries in a forum to discuss, develop, and refine economic and social policy.
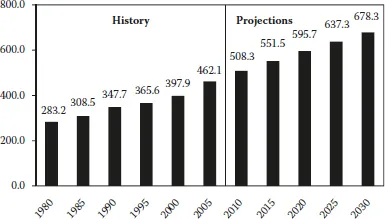
FIGURE 1.1 World marketed energy consumption 1980–2030. (Sources: History: Energy Information Administration (EIA), International Energy Annual 2006 (June–December 2008), website www.eia.doe.gov/iea. Projections: EIA, World Energy Projections Plus, 2009.) A quadrillion BTU is equal to the amount of energy in 45 million tons of coal, or 1 trillion ft3 of natural gas, or 170 million barrels of crude oil. In terms of electricity, 1 quad is equal to 293 GW h.
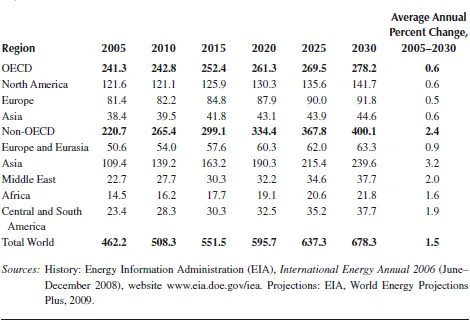
TABLE 1.1
World Marketed Energy Consumption by Country Grouping, 2005–2030 (Quadrillion Btu)
World Marketed Energy Consumption by Country Grouping, 2005–2030 (Quadrillion Btu)
The OECD consists of like-minded countries, with the 30 member states all sharing a commitment to a market economy. The organization began in 1961 as a group of European and North American nations and has since expanded to include Japan, New Zealand, Australia, Mexico, Korea, and four former communist nations, the Czech Republic, Poland, Hungary, and the Slovak Republic.
Although high prices for oil and natural gas, which are expected to continue throughout the period, are likely to slow the growth of energy demand in the long term, world energy consumption is projected to continue increasing strongly as a result of robust economic growth and expanding populations in the world’s developing countries. OECD member countries are, for the most part, more advanced energy consumers. Energy demand in the OECD economies is expected to grow slowly over the projection period, at an average annual rate of 0.6%, whereas energy consumption in the emerging economies of non-OECD countries is expected to expand by an average of 2.4% per year, as shown in Figure 1.2.
China and India are the fastest growing non-OECD economies, and they will be key world energy consumers in the future. Since 1990, energy consumption as a share of total world energy use has increased significantly in both countries. China and India together accounted for about 10% of the world’s total energy consumption in 1990, but in 2006 their combined share was 19%. Strong economic growth in both countries continues over the projection period, with their combined energy use increasing nearly twofold and making up 28% of world energy consumption in 2030 in the IEO2009 reference case. In contrast, the U.S. share of total world energy consumption falls from 21% in 2006 to about 17% in 2030.
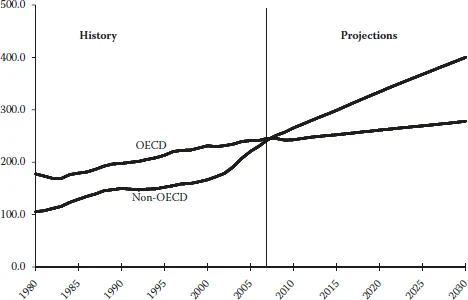
FIGURE 1.2 World marketed energy consumption: OECD and non-OECD, 1980–2030. (Sources: History: Energy Information Administration (EIA), International Energy Annual 2006 (June–December 2008), website www.eia.doe.gov/iea. Projections: EIA, World Energy Projections Plus, 2009.)
Energy consumption in other non-OECD regions is also expected to grow strongly from 2005 to 2030, with increases of about 60% projected for the Middle East, Africa, and Central and South America. A smaller increase, about 36%, is expected for non-OECD Europe and Eurasia (including Russia and the other former Soviet Republics), as substantial gains in energy efficiency result from the replacement of inefficient Soviet-era capital stock and population growth rates decline.
It almost goes without saying that clean energy sources are preferable to energy sources that pollute the environment. Although we would rather limit ourselves to wind turbines and solar cells, for practical and economic reasons these are unable to meet our needs. Accordingly, current energy demand is principally met by fossil fuels such as oil, coal, and gas. How can we best meet this growing requirement?
Contributions from renewable energy sources cannot keep pace, while the prospects for a significant contribution from nuclear energy in many countries remain clouded by social factors. So, for the time being, we will continue to be dependent on fossil fuels, as can be seen in Figure 1.3.
This includes coal, since there are probably insufficient exploitable reserves of oil and gas to keep pace for long with increasing demand. No effort should be spared, therefore, to curtail the environmental pollution that accompanies the use of fossil fuels.
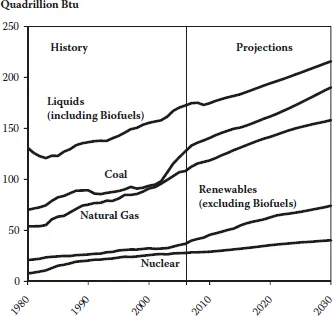
FIGURE 1.3 World marketed energy use by fuel type. (Sources: History: Energy Information Administration (EIA), International Energy Annual 2006 (June–December 2008), website www.eia.doe.gov/iea. Projections: EIA, World Energy Projections Plus, 2009.)
It is essential that we should understand how far the laws of thermodynamics can help to clarify the various processes of energy conversion, or assist us in making efficient use of energy.
Consider some examples of energy conversion, both present and future.
- Our muscular energy (as a mechanical, or work-doing, form of energy) springs from the controlled combustion of carbohydrate foods. Some of the energy is used to warm us; some is used as mechanical energy. It also seems clear that a form of electrical energy is involved at an intermediate stage. Thus life itself depends on energy conversion.
- Plant life depends on converting radiant energy from the sun into the chemical energy of sugars and carbohydrates, which are photosynthesized from water and carbon dioxide.
- Solar energy is manifested in many differen...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Preface to the Second Edition
- Preface to the First Edition
- Author
- Symbols and Abbreviations
- Chapter 1 Energy
- Chapter 2 The First Law of Thermodynamics
- Chapter 3 Thermochemistry
- Chapter 4 Spontaneous Changes
- Chapter 5 Entropy
- Chapter 6 Free Energy: The Arbiter
- Chapter 7 Chemical Equilibrium
- Chapter 8 Equilibrium Experiments and Their Interpretation
- Chapter 9 Electrochemical Cells
- Chapter 10 Free Energy and Industrial Processes
- Chapter 11 Computational Thermochemistry
- Appendix I
- Appendix II
- Appendix III
- Answers
- Suggested Further Reading
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Concise Chemical Thermodynamics by A.P.H. Peters in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.