
- 152 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Guide to Genital HPV Diseases and Prevention
About this book
Custom-designed for clinicians on the frontline of treatment and prevention of the human papillomavirus (HPV), this stand-alone handbook provides the scientific background needed to understand and administer the new HPV vaccines. Written by some of the discoverers of these vaccines, this accessible text explores the biology and epidemiology of HPV,
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Guide to Genital HPV Diseases and Prevention by William Bonnez in PDF and/or ePUB format, as well as other popular books in Medicina & Ginecologia e ostetricia. We have over one million books available in our catalogue for you to explore.
Information
Topic
MedicinaSubtopic
Ginecologia e ostetricia1
Biology
Infectious Diseases Division, Departments of Medicine, and Microbiology and Immunology, University of Rochester School of Medicine and Dentistry, Rochester, New York, U.S.A.
Division of Surgical Pathology, Department of Pathology, University of Virginia Health System, Charlottesville, Virginia, U.S.A.
1.1. Virology
1.1.1. Basic Virology
Papillomaviruses are small, round, non-enveloped DNA viruses that infect mammals, birds and reptiles, with species- and tissue-specificity. They are one of the oldest, largest, and most diverse of the known virus families. Human papillomaviruses (HPVs), like all papillomaviruses, target the stratified squamous epithelia of the body. A subset is also able to infect the glandular epithelium of the cervix.
1.1.1.1. Structure
The virion consists of a single molecule of circular, double-stranded DNA about 8 kilobasepairs in length, contained within a symmetric icosahedral protein coat, the capsid, which is made by the spontaneous assembly of the L1 major and L2 minor capsid proteins (Fig. 1.1).
1.1.1.2. Classification and Disease Association
Papillomaviruses belongs to the Papillomaviridae family. Because culture of these viruses is not readily available, taxonomy is based on genotyping and not serotyping, which is traditionally used in virology. Genotypes are considered distinct if they share less than 90% homology in the DNA sequence of the open reading frame (ORF), coding for the major capsid protein. Subtypes have between 90% and 95% homology, and variants between 96% and 98%. The Papillomaviridae family has 18 genera. The human papillomaviruses belong to the Alpha-, Beta-, Gamma-, Mu-, and Nupapillomavirus. They are numbered in order of discovery.
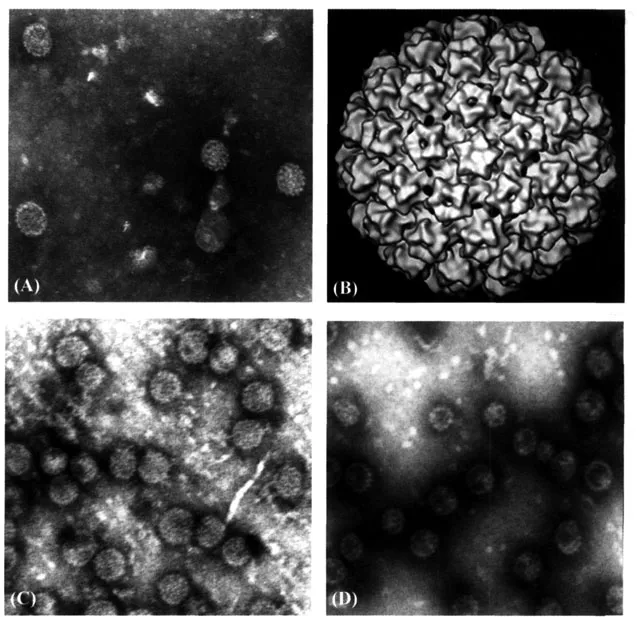
Figure 1.1 Papillomavirus capsid structure. (A) Electron micrograph of native HPV11 virions (Courtesy W. Bonnez); (B) cryoelectron micrograph of a BPV1 virion; (C) electron micrographs of HPV16; and (D) HPV18 L1 virus-like particles (VLPs). Source: For parts A, B: http://commons.wikimedia.org/wiki/lmage:Papillomavirus_capsid.png. For parts C, D: Source: Rose RC, Bonnez W, Da Rin C, McCance DJ, Reichman RC. Serological differentiation of human papillomavirus types 11, 16 and 18 using recombinant virus-like particles. J Gen Virol 1994; 75:2445–2449.
At least 111 HPV have been officially recognized, but this is rapidly changing, and the actual number is thought to be considerably higher. Although different HPVs infect different anatomic sites and have different disease-associations (Table 1.1), many of the most recently identified HPV genotypes do not have a clear pathogenic role, and do not appear in Table 1.1. Although not completely reflective of phylogeny, it is convenient to classify HPVs into three groups according to associated diseases. Members of the first group of HPVs are responsible for the very common cutaneous warts (hand, plantar, and flat warts). These viruses are found only rarely in the genital tract. Members of the second group are found in a rare genodermatosis, epidermodysplasia verruciformis, whereby associated lesions have a high propensity in adulthood to develop into squamous cell cancers in the sun-exposed areas of the body. These viruses are also frequently present in the normal skin.
Table 1.1 HPV Types and Disease Associations
Disease | Frequent association | Less frequent association |
Cutaneous warts | 1, 2, 4 | 3, 7, 10, 26, 27, 28, 29, 38, 41a, 49, 57, 63, 65, 75, 76, 77, 80, 83, 84, 86, 87 |
Epidermodysplasia verruciformis | 5, 8, 9, 12, 14, 15, 17 | 19, 20, 21–25, 36–38, 47, 49, 50, 93 |
Condylomata acuminata | 6, 11 | 30, 42, 43, 44, 45, 51, 54, 55, 70 |
Intraepithelial neoplasias | 6, 11, 16, 18 | 30, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51–53, 56, 57, 58, 59, 61, 62, 64, 66, 67, 68, 69, 71, 72, 74, 82 |
Carcinomas | 16, 18 | 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 67, 68, 70, 73, 82 |
aThe genotypes in bold have an established or possible oncogenic potential.
The third group is made of the genital HPVs, also called mucosal HPVs, because they infect the mucous membranes of not only the anogenital tract, but also of the upper aerodigestive tract. The genital papillomaviruses belong to the Alphapapillomavirus genus. Their phylogeny is shown in Figure 1.2. Within the genus, different species are recognized, each with a representative genotype. For the purpose of this book, it is important to recognize that HPV-6 is the representative type of species 10, to which HPV-11 also belongs; HPV-16 is the representative of species 9, and HPV-18 of species 7. What accounts for tissue tropism is not well understood.
1.1.1.3. Papillomavirus Genomic Organization
Viral open reading frames are arrayed in a linear fashion on only one strand of the double-stranded circular DNA genome (Fig. 1.3). Viral genome functioning is controlled by the so-called “upstream regulatory region” (URR), which contains many binding sequences for cellular and viral factors that alone or in concert orchestrate the selective synthesis of viral messages and the replication of the viral genome. Genomic organization is well conserved among all HPVs, with Early (“E”) and Late (“L”) ORF regions that are capable of coding viral proteins, following the URR. (Fig. 1.3). The names Early and Late refers loosely to when the messages and proteins from these ORFs appear during viral infection.
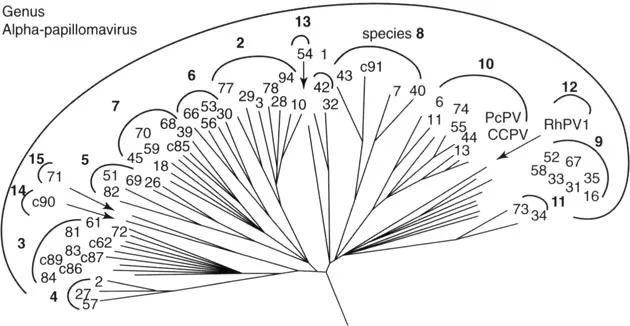
Figure 1.2 Phylogeny of the Alphapapillomavirus genus. Source: From de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004; 324:17–27.
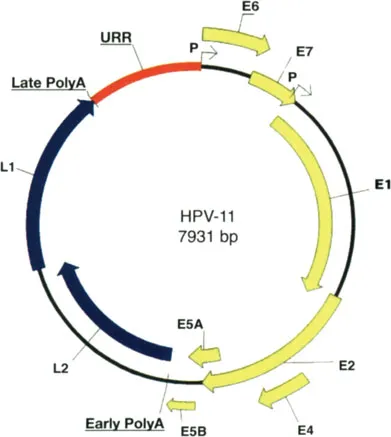
Figure 1.3 Genomic organization of a representative papillomavirus genotype (HPV-11).
Table 1.2 HPV Viral Proteins Main Functions
Proteina | Function(s) | |
“Early” proteins | E1 | Viral DNA replication Maintenance of episomal state Control of gene transcription |
E2 | Control of viral transcription and DNA replication Inhibition of E6 and E7, and activation of E2 N-terminus gene expression | |
E4 | Not well defined, but interact with the cellular intermediate filaments | |
E5 | Enhances growth factor effects Immune evasion | |
E6b | Destroys p53, a tumor suppressor protein that represses the cell cycle Inhibits apoptosis Contributes to immune evasion | |
E7b | Inactivates the retinoblastoma protein, a tumor suppressor protein that represses the cell cycle Contributes to immune evasion | |
“Late” proteins | L1 | Major capsid structural protein, pentamer/capsid self-assembly |
L2 | Minor capsid structural protein (virion assembly and infectivity) |
aThere is no E3 protein.
bThe interaction of E6 and E7 with tumor suppressor proteins is limited to high-risk HPVs.
1.1.1.4. Viral Proteins
Viral ORFs in both the E and L regions are named according to decreasing size. Thus, E1 and L1 are the largest of the E and L region viral proteins, respectively. The main known and suspected functional properties of the various viral proteins are listed in Table 1.2.
1.1.1.5. Growth in Cell Culture and Animal Model Systems
Due to strict species- and tissue-specificities of these viruses and their requirement for differentiating epithelium for completion of the viral life cycle, growth of HPV genotypes in the laboratory for a long time was impossible, and remains difficult and complex. This is why for research purposes, one still relies on animal papillomavirus models such as the cottontail rabbit papillomavirus, the bovine papillomavirus, and the canine oral papillomavirus. HPV-1, then HPV-11, and later HPV-16 were first grown, beginning in 1985, by infecting small fragments of human epithelial tissue (mostly neonatal foreskin), and implanting them under the renal capsule of immunodeficient mice (athymic “nude” mice or animals with the severe combined immunodeficiency syndrome). In this type of model the viral infection recapitulates the macroscopic, microscopic, and molecular features of a natural infection. It has been possible since, to grow HPV in skin organotypic (artificial skin) culture systems.
1.1.1.6. Antigenicity
All HPV proteins are immunogenic and thus capable of eliciting both humoral and cellular immune responses. In natural infection, tight regulatory control over viral gene expression acts to minimize antigen exposure in the infected host; thus, the magnitude of such responses usually is quite low. Although both the E6 and E7 proteins of the high-risk HPVs are continually expressed in neoplastic lesions, E6-specific cellular responses are more often associated with disease resolution than are similar responses against the viral E7 protein. The L1 major capsid protein displays a common (shared across all papillomaviruses tested) linear epitope when denatured that usually is not seen by the immune system in natural infection. By contrast, the L1 protein in its native conformation is immunogenic. It can readily assemble into an empty capsid in the absence of L2, the minor capsid protein. This empty capsid when made in vitro is called a virus-like particle (VLP) and is the basis of the current vaccine (Fig. 1.1). These VLPs have the same immunologic properties as the infectious virions. They possess immunodominant antigenic sites that generate a strong binding and neutralizing antibody response that is generally genotype-specific. The second structural and functional component of the viral capsid, the L2 minor capsid protein, also possesses neutralizing epitopes within the amino-termina...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Preface
- Contributors
- Introduction
- 1. Biology
- 2. Epidemiology
- 3. Diseases
- 4. Treatment
- 5. Diagnosis
- 6. Screening
- 7. Prevention
- Index