![]()
1
Neuroimaging of Healthy Cognitive Aging
Nancy A. Dennis
Roberto Cabeza
Duke University
Cognitive aging research and theory has, until recently, been based upon behavioral measures of cognitive performance such as response time and accuracy. Results from behavioral methodologies have indicated a general age-related decline in cognitive functions such as speed of processing, attention, perception, working memory, and cued and free recall—and age invariance when assessing cognitive processes associated with vocabulary and semantic memory. Recently, advances in the area of neuroimaging have allowed for the examination of the relationship between cognitive and neural differences in the aging brain. Given that cognitive processes depend on brain anatomy and physiology, it is natural to expect that previously observed behavioral differences in aging are intimately linked to age-related changes in the integrity of cerebral architecture and function.
By using in vivo neuroimaging techniques such as positron emission tomography (PET) and magnetic resonance imaging (MRI), researchers can tap into the neural substrates of cognitive aging linking behavior and function. As this technology has improved over the last two decades, significant advances have been made in the field of functional neuroimaging of cognitive aging. Research has shown us that, despite a common notion that everything declines in aging, neural activity associated with cognitive aging is characterized by both age-related increases as well as age-related decreases in brain activity. Failure on the part of older adults to activate brain regions typically recruited by younger adults during cognitive tasks is usually characterized as neurocognitive decline. However, additional neural recruitment by older adults during task performance, beyond that seen in younger adults, is typically characterized as functional compensation. Examination of both types of neural activity is necessary for developing a better understanding of the plasticity of both the aging brain and cognitive aging in general.
There are two basic neuroimaging approaches to link the effects of aging on the brain and on behavior. The first is to correlate a resting neuroimaging measure, such as an MRI measure of brain volume or a PET measure of resting blood flow, to a behavioral measure obtained outside the scanner, such as performance in a memory test. The second is to use a task-related neuroimaging technique, such as functional MRI (fMRI), to measure activity in the scanner while participants are performing a cognitive task. Both approaches have strengths and weaknesses and complement each other. In the first major section of this chapter, we provide a brief overview of resting neuroimaging studies of aging, and in the second major section, which is the core of the chapter, we review functional neuroimaging studies of aging in various cognitive domains. The chapter ends with a section linking consistent neuroimaging findings to major theories of cognitive aging.
Resting Neuroimaging Studies of Aging
Resting neuroimaging measures include measures of brain volume, white matter integrity, resting blood flow and metabolism, and neurotransmitter function. These different measures are considered in separate sections below.
Measures of Brain Volume
Understanding age-related atrophy is essential to the understanding of functional differences between age groups. However, as it is not the main focus of this chapter, only a very brief overview of volumetric MRI studies of healthy aging is presented here (for a more complete review of age-related structural decline see Raz, 2005). While earlier studies of structural differences in aging have used a cross-sectional approach, more recent studies have used a longitudinal design. Despite this inherent bias towards healthier and more stable samples, longitudinal estimates of decline usually exceed those of cross-sectional studies. One explanation is that in cross-sectional analyses, intrapersonal change is masked to some degree by the noise associated with age-independent individual differences, whereas longitudinal studies are able to exclude both individual differences and cohort effects (Raz et al., 2005). The current section will focus on these more recent assessments of gray matter change across time.
Changes in whole brain volume as a function of aging have been examined in over 14 studies to date (Raz, 2005). In general, these changes are not linear, but become steeper in old age. For example, the cerebral cortex as a whole declines at a rate of 0.12% per year in younger adults but at a rate of 0.35% per year in adults over 52 years of age. Similarly, ventricles expand at a rate of 0.43% in younger adults but at a rate of 4.25% after the age of 70.
From a cognitive neuroscience perspective, the most interesting finding is that age-related atrophy differs across regions. With an average decline rate of between 0.9% and 1.5% per year, the frontal lobes show the steepest rate of atrophy (Pfefferbaum, Sullivan, Rosenbloom, Mathalon, & Lim, 1998; Raz et al., 2005; Resnick, Pham, Kraut, Zonderman, & Davatzikos, 2003). Moreover, frontal atrophy has been shown to correspond with cognitive deficits mediated by frontal regions. For example, Gunning-Dixon and Raz (2003) found that in a large group of older adults perseveration errors on the Wisconsin Card Sorting Task, a measure of executive functioning, negatively correlated with prefrontal volume.
The parietal lobes show the second steepest decline in function (Pfefferbaum et al., 1998; Raz, 2005; Resnick et al., 2003), with an annual rate between 0.34 and 0.90%. Compared to frontal and parietal lobes, the occipital lobe shows small or nonsignificant age-related atrophy. Additionally, atrophy rates also differ among subregions of each lobe. For example, there is evidence that within frontal and parietal cortex, more inferior subregions show the steepest rates of decline (Resnick et al., 2003).
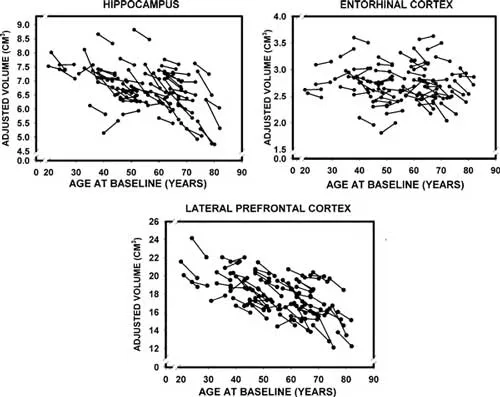
Due to their role in memory function, the medial temporal lobes (MTL) have elicited more focal examinations over the years. Like other brain regions, longitudinal estimates of temporal lobe shrinkage exceed those of cross-sectional data (Scahill, Frost, Jenkins, Whitwell, Rossor, & Fox, 2003). Additionally, subregions of the temporal lobes (e.g., entorhinal cortex, hippocampus, parahippocampal gyrus) exhibit differential rates of decline. For example, a recent longitudinal study found that in healthy older adults, the hippocampus showed substantial atrophy whereas the entorhinal cortex did not (Raz et al., 2005). Furthermore, studies have shown that the rate of hippocampal atrophy increases with age (Raz, Rodrigue, Head, Kennedy, & Acker, 2004; Scahill et al., 2003). In one study, for example, this rate was an average of 0.86% per year in the whole sample (26–82 years) but 1.18% when considering only individuals over 50 years of age (Raz et al., 2004). A review of 12 studies estimated that after the age of 70 this rate may be as high as 1.85% per year (see Raz, 2005). These findings are very interesting because the entorhinal cortex is one of the regions first affected by Alzheimer’s disease (AD; Braak, Braak, & Bohl, 1993). As discussed later, together with recent fMRI evidence of dissociations between hippocampal and rhinal functions in aging (Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006), these findings have implications for the early diagnosis of AD. (See Figure 1.1.)
Fig. 1.1. Longitudinal changes in adjusted volumes of the hippocampus, entorhinal cortex, and lateral prefrontal cortex as a function of baseline age. Reproduced by permission of Oxford University Press from Raz et al. (2005). Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex, 15(11), 1676– 1689.
Correlating structure with function, Rodrigue and Raz (2004) acquired both volumetric measures of the prefrontal cortex (PFC), hippocampus, and entorhinal cortex and measures of episodic memory across a 5-year interval in a large group spanning 26–83 years of age. While the volume of hippocampus and PFC correlated with age at baseline and follow-up, once the effects of age were controlled for, neither predicted memory performance. However, increased shrinkage of entorhinal cortex was associated with poorer memory performance at follow-up. Results support previous work showing a correlation between entorhinal cortex shrinkage and memory performance in the very old (Du et al., 2003). Additionally, Persson and colleagues (2006) found reduced hippocampal volume in a group of older adults whose episodic memory performance declined across a decade compared to that of a group whose memory performance remained stable
Subcortical atrophy in healthy aging is also prevalent, with longitudinal studies showing age-related striatal decline beginning in early adulthood. Four studies assessing decline in younger adults (average age = 29 yrs) showed caudate shrinkage that exceeds 1% per year (Chakos et al., 1994; Lang et al., 2001; Lieberman et al., 2001; Tauscher-Wisniewski, Tauscher, Logan, Christensen, Mikulis, & Zipursky, 2002) (but see DeLisi, Sakuma, Tew, Kushner, Hoff, & Grimson, 1997). Only one study to date has assessed striatal shrinkage in a group that included older adults. Following 53 adults (ranging in age from 26 to 82 at time 2) over five years, researchers see a more modest 0.83% decline in caudate volume per year (Raz et al., 2003). However, caudate decline is not representative of decline in other striatal nuclei. When assessed separately, the caudate exhibits a steeper rate of decline compared to the putamen, which shows less decline than the globus pallidus (Lang et al., 2001; Raz, Rodrigue, Kennedy, Head, Gunning-Dixon, & Acker, 2003). When correlating motor speed and striatal atrophy in a large sample of older adults (129 older adults ranging in age from 64 to 74 years), Soderlund, Nyberg, and Nilsson (2004) concluded that atrophy in the caudate nucleus predicted poorer performance in women, but not men.
Several metencephalic structures (e.g., cerebellum, vermis, pons) also show decline with age (Raz, 2005). While most longitudinal studies in this area have been conducted within a small age range (e.g., either younger or older adults), they generally show significant age-related shrinkage across all metencephalic structures. On average the cerebellum shows the greatest shrinkage, followed by the vermis, with the pons showing the smallest decline.
Finally, age-related decline has also been seen within the corpus callosum. Though cross-sectional studies show only modest shrinkage in the corpus callosum (Driesen & Raz, 1995), longitudinal studies show more significant age-related reductions (e.g., Sullivan, Pfefferbaum, Adalsteinsson, Swan, & Carmelli, 2002; Teipel et al., 2002), around 0.90% per annum. Furthermore, thinning of the corpus callosum over a 4-year period has been shown to correlate with performance on the Stroop task (Sullivan, Pfefferbaum et al., 2002) in older adults. A later study by Sullivan, Adalsteinsson, and Pfefferbaum (2006) found that fronto-callosal fibers showed a steeper rate of age-related decline than posterior fibers. Results support the view that age-related callosal degradation contributes to functional decline and that anterior regions are more vulnerable than other regions.
Interestingly, gender differences in volumetric decline across time appear nonexistent (Raz et al., 2005; Resnick et al., 2003), except perhaps in the caudate (Raz et al., 2005) which may show greater decline in women compared to men. There is also some evidence that volume loss is attenuated (though not absent) in the healthiest of individuals (Resnick et al., 2003). Overall, results support differential aging of individual cortical and subcortical regions.
Measures of White Matter Integrity
Postmortem studies suggest that age-related white matter loss occurs throughout the brain, and in particular in the frontal lobes, with white matter loss more extensive than gray matter loss (Double et al., 1996; Esiri, 1994; Kemper, 1994). In contrast, in vivo studies find no significant loss in healthy aging (Raz, 1996; Raz et al., 1997; Sullivan, Marsh, Mathalon, Lim, & Pfefferbaum, 1995), albeit some evidence suggests loss restricted to frontal regions (Raz et al., 1997; Salat, Kaye, & Janowsky, 1999). Several studies examining white matter differences across the entire lifespan (Courchesne et al., 2000; Pfefferbaum, Mathalon, Sullivan, Rawles, Zipursky, & Lim, 1994; Sullivan, Rosenbloom, Serventi, & Pfefferbaum, 2004) show decline beginning only in the seventh decade (but see Liu & Cooper, 2003).
Beyond overall volume measurements, it is also possible to measure white matter integrity. One method is to assess the number of white matter hyperintensities (WMHs) in the aging brain. While the exact cause of WMHs is unknown, they are posited to arise both from neural and vascular pathologies (for a review see Pantoni & Garcia, 1997). A review of 49 studies of healthy aging found the mean correlation between age and severity of WMHs to be .37 (Gunning-Dixon & Raz, 2000). Other risk factors for WMHs include hypertension (Gunning-Dixon & Raz, 2000), elevated systolic blood pressure (DeCarli et al., 1995), and apolipoprotein E-4 (e.g., Kuller et al., 1998; but see Schmidt et al., 1996).
In addition to correlating prefrontal volume with executive functioning, Gunning-Dixon and Raz (2003) also found that WMHs in the prefrontal cortex are independently associated with perseveration errors on the Wisconsin Card Sorting Task. Additionally, in an extensive study including 1254 participants ranging in age from 64 to 76 years, Soderlund, Nyberg, Adolfsson, Nilsson, and Launer (2003) found that periventricular WMHs and subcortical atrophy predicted lower performance on both motor speed and the Stroop task. Results remained stable after controlling for demographic factors (e.g., age, gender, education).
An advent of recent years, diffusion tensor imaging (DTI) has been employed to identify the structural integrity of white matter tracts in the brain. DTI measures assess changes in the MR signal due to the movement of water molecules. In healthy myelinated fiber tracts, water molecules travel along the internal membrane, not across the fiber walls. With degradation of myelin sheath in normal aging, the probability and speed of diffusion along the fiber walls diminish. Two measures, fractional anisotropy (FA) and apparent diffusion coefficient (ADC), quantify these changes and, in turn, white matter integrity. Reduced FA and increased ADC are indicative of degraded micro-structural tissue integrity.
Several studies have investigated age-related differences in FA and ADC in numerous brain regions (Abe et al., 2002; Madden, Whiting, Huettel, White, MacFall, & Provenzale, 2004; Madden et al., 2007; Pfefferbaum, Sullivan, Hedehus, Lim, Adalsteinsson, & Moseley, 2000; Salat et al., 2005; Sullivan et al., 2001). Significant age-related decline in white matter integrity was found in the centrum semiovale (white matter in the medial 55% of the left and right hemispheres), frontal white matter tracts, the posterior limb of the internal capsule, and the genu of the corpus callosum. Furthermore, evidence suggests that age-related decline in anisotropy and diffusivity is more pronounced in anterior, as opposed to posterior, regions. Correspondingly, two studies focusing specif...