
eBook - ePub
Handbook of Biomedical Fluorescence
- 694 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handbook of Biomedical Fluorescence
About this book
Melding basic and clinical science, this reference provides a comprehensive overview of the roles that biophysics, photochemistry, and computational modeling play in the biomedical applications of fluorescence spectroscopy and imaging. Penned by pioneering researchers, the Handbook of Biomedical Fluorescence discusses fundamental aspects of fluorescence generation in organic molecules within tissue, theoretical and experimental views of how light propagation in tissue can be used to interpret fluorescence signals, endogenous and exogenous fluorescence agents in medical or basic research studies, and radiation transport, diffusion theory, and the Monte Carlo method.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Handbook of Biomedical Fluorescence by Mary-Ann Mycek, Brian W. Pogue, Mary-Ann Mycek,Brian W. Pogue in PDF and/or ePUB format, as well as other popular books in Medicine & Biochemistry in Medicine. We have over one million books available in our catalogue for you to explore.
Information
1
Introduction to Fluorescence and Photophysics
Massachusetts General Hospital, Boston, Massachusetts, U.S.A.
1. ABSORPTION AND FLUORESCENCE
Emission of light in the form of fluorescence often accompanies deactivation of an electronically excited species. Fluorescence is defined as the radiative transition between two electronic states of the same spin multiplicity. Most organic molecules have “paired” electrons in their ground state molecular orbital configuration. The spins are balanced (e.g., and , S = Σs = 0) and the spin multiplicity (Ms = 2S + 1 = 1) is singlet. Alternatively, inversion of the spin of the excited electron results in the two unpaired electrons having the same spin orientation. The overall spin S is 1 , the spin multiplicity (Ms = 2S + 1) is 3, and a triplet state results.
Most commonly, fluorescence refers to singlet-singlet transitions, especially the transition between the lowest, or first, excited singlet state (S1) and the ground state (S0). Other types of less common fluorescence processes do occur, such as that from the second (S2) excited singlet state, and the doublet-doublet fluorescence exhibited in the radiative relaxation between excited and ground state free radicals (one unpaired electron, , Ms = 2S + 1 = 2). However, this introduction will focus on S1-S0 fluorescence, which is by far the most common type.
The relationship between absorbance and fluorescence can be illustrated using simple potential energy diagrams of the type shown in Fig. 1 that show the relative electronic and vibrational energy levels as a function of internuclear separation in the affected bond.
The absorption process involves interaction of the molecule in the ground state with a photon to promote an electron from a lower energy to a higher energy molecular orbital. The absorbance (A) of a sample is proportional to the concentration (c, in molarity M; Beer’s law) of absorbing species in a sample of pathlength ℓ (cm) traversed by the light and is generally independent of the intensity of the excitation light (Lambert’s law), although the latter may not hold under high-intensity laser irradiation. In transparent media the pathlength is simply the thickness of the sample, but it is more complex to determine in opaque or highly scattering materials. This results in the common expression of the Beer-Lambert law shown in Eq. (1). The molar absorption coefficient (ε in M−l cm−1) is the proportionality factor and its magnitude reflects the probability of the absorption of a photon of a given energy by the molecule. The absorption spectrum of a compound is constructed by plotting ε as a function of excitation wavelength.
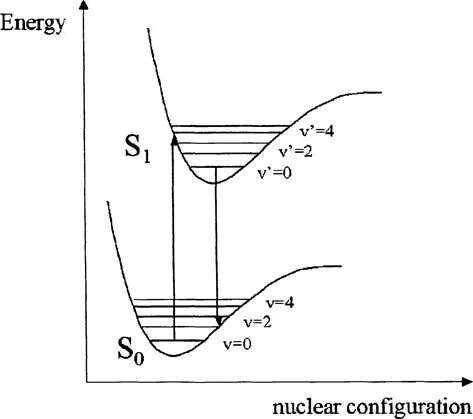
FIGURE 1 Potential energy diagram showing absorption and emission transitions between vibrational sublevels in ground and electronically excited states.
(1) |
The absorption process takes place on a time scale (~10−15 sec) much faster than that of molecular vibration; thus, absorption occurs in a “vertical” manner, i.e., the internuclear geometry will be identical immediately before and after absorption to form the excited state. This is the Franck-Condon principle. In the excited state, the electron is promoted to an antibonding orbital such that the bond order is reduced, the atoms in the bond are less tightly held, and the equilibrium bond length is subsequently longer. This is shown as a displacement to the right of the excited state potential curve with respect to the ground state in Fig. 1. The vibrational level that is initially populated is that where vertical overlap at the energy of the absorbed photon occurs and is generally v′ > 0. In Fig. 1 the absorption is shown to the v′ = 3 level of the S1 state. The simplest fluorescence in terms of photophysics would be the exact reverse of the absorption process, emitting light of a wavelength identical to that absorbed. This is termed “resonance” fluorescence. In Fig. 1 this would correspond to a transition from the v′ = 3 level of the S1 state back to the v = 0 level of the S0 state. Such fluorescence can be observed from atoms or molecular gases at very low pressures but is not usually apparent for larger molecules in condensed phases such as liquids and solids. This is due to the fact that vibrational deactivation, through intermolecular collisions, occurs more rapidly than the fluorescence emission process, with the result that fluorescence generally occurs from the lowest vibrational level (v′ = 0) of the electronic excited state.
The transition that is associated with the emission of a photon is also so rapid that no change in nuclear configuration can occur during the process. The Franck-Condon principle again dictates that the vibrational level that is initially populated in the electronic ground state will be that which shows “vertical” overlap with the v′ = 0 level of the S1 state. This would be the v = 1 level in the example shown in Fig. 1. Thus, the energy of the emitted photon will be significantly lower than the absorbed photon and the fluorescence is red shifted with respect to absorption.
Several other points are worth noting from this diagram. The energy level spacings between adjacent vibrational levels decreases with increasing energy, and a similar spacing of vibrational levels is often seen in the ground (S0) and excited (S1) states. This results in the “mirror image” relationship commonly observed between absorption and emission spectra. This is shown in more detail in Fig. 2. The electronic excited state energy of S1 can be obtained from the transition between the υ = 0 levels in both states and is termed the 0-0 transition. Due to slight changes in internuclear distances at equilibrium in both states, the 0-0 transition energy (E0-0) is often not identical in absorbance and fluorescence but can be estimated from the wavelength at which these two spectra overlap. This difference is also termed the Stokes shift. The Stokes shift and E0-0 estimation are shown in Fig. 3 for the example of the photodynamic therapy (PDT) agent, benzoporphyrin derivative monoacid ring A (BPDMA).
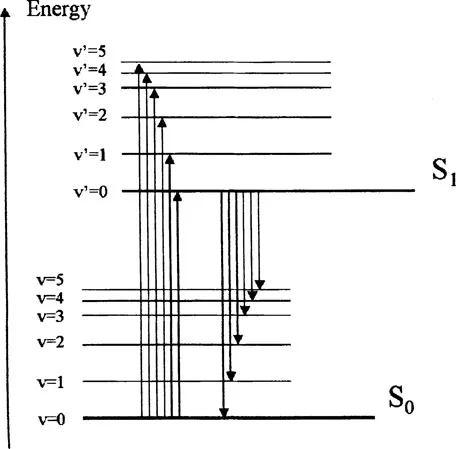
FIGURE 2 Possible absorption and emission transitions between vibrational levels in ground and excited states. Emission occurs at lower energy and is red shifted with respect to absorption bands.
2. DEACTIVATION OF THE S1 STATE
Fluorescence is only one of the possible mechanisms by which an excited molecule can undergo relaxation to the ground state. The Jablonski diagram in Fig. 4 shows that there are a number of potential transitions open to the S1 state after population by excitation and internal conversion from upper states. Here the formalism of a solid arrow depicting a radiative transition and a wavy arrow depicting a nonradiative transition has been followed. The molecule can undergo both nonradiative (internal conversion, ic) and radiative (fluorescence) relaxation to the ground state (S0) or nonradiative transition (intersystem crossing, isc) to the lowest excited triplet state (T1).
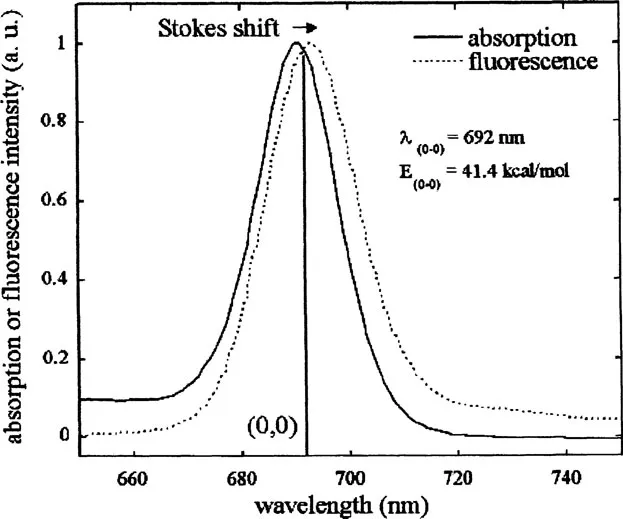
FIGURE 3 Lowest energy absorption ban...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Contributors
- 1. Introduction to Fluorescence and Photophysics
- 2. Diffusion Modeling of Fluorescence in Tissue
- 3. Monte Carlo Simulations of Fluorescence in Turbid Media
- 4. Intrinsic Fluorescence Spectroscopy of Biological Tissue
- 5. Real-Time In Vivo Confocal Fluorescence Microscopy
- 6. Two-Photon Microscopy of Tissues
- 7. Fluorescence Lifetime Imaging Microscopy of Endogenous Biological Fluorescence
- 8. Survey of Endogenous Biological Fluorophores
- 9. Cervical Dysplasia Diagnosis with Fluorescence Spectroscopy
- 10. Fluorescence Spectroscopy and Imaging for Skin Cancer Detection and Evaluation
- 11. Lung Cancer Imaging with Fluorescence Endoscopy
- 12. Time-Resolved Laser-Induced Fluorescence Spectroscopy for Staging Atherosclerotic Lesions
- 13. Applications of the Green Fluorescent Protein and Its Variants in Tumor Angiogenesis and Physiology Studies
- 14. Near-Infrared Imaging with Fluorescent Contrast Agents
- 15. Fluorescence in Photodynamic Therapy Dosimetry
- 16. Controlled Drug Delivery in Photodynamic Therapy and Fluorescence-Based Diagnosis of Cancer
- 17. Tissue Oxygen Measurements Using Phosphorescence Quenching
- Index