Interest in hypnosis as a potential research tool in the study of sleep has been sparked by the compelling similarities between the two states. In this report, an attempt will be made to evaluate three broad issues. First, to what extent is there a physiological similarity between hypnosis and sleep?
Phenomenological Similarities between Hypnosis and Sleep
If he had not witnessed the induction procedure, the casual observer might well characterize a typical hypnotized S as being asleep. It was this sleeplike appearance that led Braid (1852) to coin the terms “hypnosis” from the Greek hypnos [to sleep] and “somnambulist” from the Latin somnus [sleep] and ambulae [walk], to describe the deeply hypnotized person. According to Braid, hypnosis was an artificially induced state of somnambulism.
There are many parallels between sleep and hypnosis. The hypnotized person often appears to be asleep, and he typically describes the experience as sleeplike. When awaking from either condition, the person remembers little of what has transpired. Like the sleepwalking somnambulist, the hypnotized person may move about and talk, and he maintains contact with selected aspects of the external world. Some parallels exist in cognitive processes. Vivid dreams may occur in both states. The sleepwalker avoids obstacles; the hypnotized subject avoids colliding with a chair he is negatively hallucinating. The long historical association between hypnosis and sleep is still reflected in many of the standard induction suggestions that S should enter into a deep, relaxed, restful sleep.
The relationship between hypnosis and sleep has intrigued scientists throughout the history of hypnosis. The interest in the interrelationship between the two conditions reached its culmination in Pavlov’s theoretical position, which is particularly influential in Eastern Europe. Sleep is considered by Pavlovian theorists as a state of cortical inhibition, while hypnosis is more or less halfway between sleeping and waking, a state of partial excitation surrounded by cortically irradiating inhibition. This viewpoint has been reviewed and evaluated recently by Edmonston (1967; see also Chapter 13 of this book).
The phenomenological similarities between sleep and hypnosis raise many interesting theoretical and methodological questions. Phenomena that appear similar may indeed have many different qualities. Hypnosis has a chameleonlike character that precludes easy determination of its essential features. The somnambulistic state studied by Braid was already quite different in appearance from animal magnetism as practised by Mesmer. For Mesmer, sleep was an aftereffect of the crises, or hysterical seizures, of his patients. Many effects that have temporarily gained vogue as invariant characteristics of hypnosis can be attributed to the influence of culturally adetermined factors and the expectations of the hypnotized S. New phenomena of hypnosis have been “invented” by subtle manipulations of S’s expectations. Orne’s (1959) demonstration of catalepsy of the dominant hand provides a dramatic example of the “discovery” of an apparently new hypnotic phenomenon. In spite of the elusive nature of the hypnotic state and the difficulty of establishing its invariant effects, its existence as a phenomenon has been challenged by only a few modern investigators.
The relationships between sleep and hypnosis have been further obscured by the apparent interchangeability of the two states.1 If the hypnotized S is left alone, or if specific suggestions are given, he may pass into a natural sleep. Similarly, in the context of hypnotic research, it appears that a sleeping S may sometimes awake directly into a hypnotic state rather than into a normal waking state, particularly if he has been instructed to do so before falling asleep. Whether the individual is in a sleep, hypnotic, or normal state at a given time may depend upon how he perceives what he is expected to do. The precise state in which an individual exists at any given time is extremely difficult to evaluate by objective methods. Although there are several objective behavioral and physiological characteristics that help identify S’s present state, S’s verbal description of the subjective aspects of his experiences ultimately provides the main criterion for determining his state.
Characteristics of the Waking-Sleep Cycle
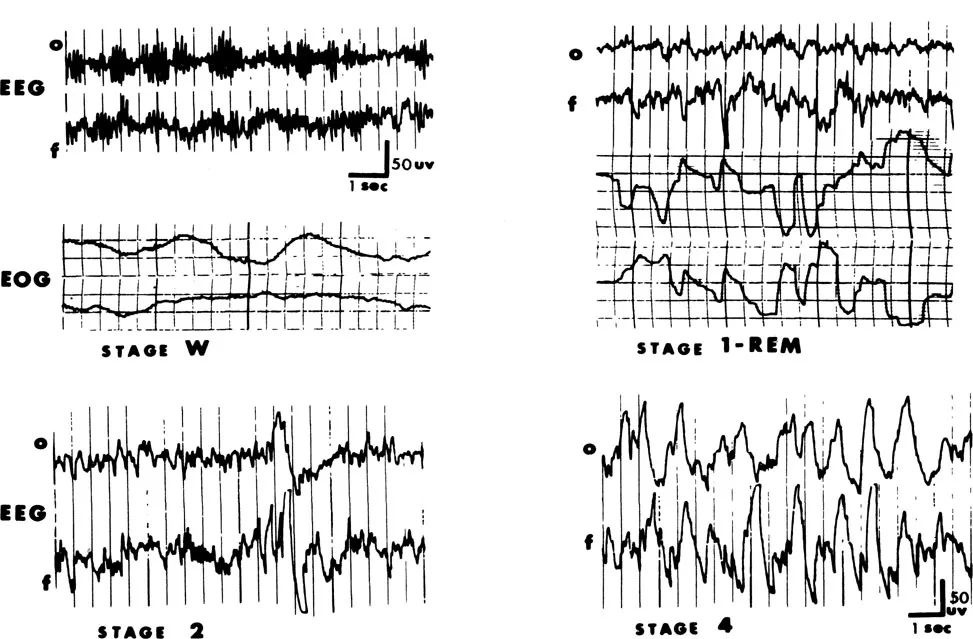
Behaviorally, sleep is easily recognized. The behavioral evidence for sleep includes the person’s general appearance, physical relaxation, lack of communication with and response to the external world, and special manifestations such as snoring. The sleeping S is prone and relaxed, his breathing is slow and even, and autonomic functions are depressed. Sleep can be confirmed post hoc by the person’s subjective experience. The S reports he has not been particularly aware of anything, he usually has a poor sense of elapsed time, and he may include in his description reports of dreaming. The subjective awareness of sleep is a universal experience, and is easily described. Following the development of electronecephalographic (EEG) technology, physiological indices were developed that allowed a more stringent definition of sleep. A variety of physiological and biochemical changes during sleep have been studied extensively. The behavioral, subjective, and physiological signs of sleep are usually, although not always, in close agreement (Dittborn & O’Connell, 1967). Sleep has commonly been considered a relatively homogeneous experience. Both subjectively and behaviorally, sleep seems to be much the same throughout a typical eight-hour night. The EEG evidence, however, shows clearly that sleep is complex, consisting of two or more separate states that occur in predictable cycles both in humans and in many species of animals. These stages are quite different physiologically, and the accompanying behavioral and cognitive activity associated with them may be different. The salient characteristics of human sleep stages (Rechtschaffen & Kales, 1968) are described below. The discussion of EEG patterns during waking and sleep (and during hypnosis) requires that special attention be paid to one particular EEG rhythm—alpha activity of 7-13 cycles per second (Hz.). Illustrative samples of occipital and frontal EEG recordings and horizontal eye movement recordings are presented in Figure 6.1.
Waking (eyes closed). The EEG will usually show alpha activity intermixed with low-voltage, mixed frequency activity. During on-line recording, alpha occurs predominantly in the occipital regions. It may occur continuously for many seconds or intermittently in “waves” or “envelopes” of a few seconds’ duration. Some individuals rarely generate alpha; some do so almost continuously. Under relatively controlled, optimal conditions, the distribution of the amount of alpha (density) in a homogeneous sample is approximately normal. When alpha is present, its density within a given individual varies considerably over time, depending, in part, on what S is doing. Alpha is most likely to occur when the person is relaxed, with his eyes closed, and when he is not engaging in any particular mental activity. Complex cognitive activity may block alpha. Alpha also disappears as the person becomes drowsy. The paradox of alpha activity is that its density decreases both with drowsiness and with heightened arousal or difficult cognitive tasks. In both instances it is replaced by similar, mixed, low-voltage fast activity. The sudden appearance of alpha activity in an otherwise “flat” random record may indicate arousal if the person has been asleep, or it may indicate the onset of drowsiness if the individual has been engaged in an attentive task.
Changes in alpha density must be interpreted cautiously and only when it is known what S has been doing. If the waking EEG does not contain alpha, it is indistinguishable from the EEG during sleep stage 1 and stage REM, described below. For those individuals who show little or no alpha even under optimal waking conditions, there is no way to discriminate from the EEG alone whether the person is aroused, relaxed, drowsy, or in stage 1 or stage REM sleep. If Ss without waking alpha are not excluded from samples, critical determinations of Ss’ position on the arousal (and sleep) continuum cannot be made. It has not always been reported whether samples contain some nonalpha generators.
FIGURE 6.1 Electrophysiological recordings of sleep stages. Occipital and frontal EEG and horizontal eye movements (EOG) are shown for waking and stage REM. For stages two and four, only the EEG is shown.
Stage 1. Within the limits of his optimal waking alpha density, as S falls asleep alpha becomes intermittent and lower in amplitude, until it disappears. Desynchronized fast activity dominates the EEG record, which is similar to the activated waking record. Slow, rolling eye movements (SEM) are usually observed. This descending stage 1 recurs during the night whenever S falls back to sleep after awakening. If aroused during this descending stage, he often denies he was asleep, typically reporting that he was thinking, daydreaming, or engaging in hypnogogic reverie.
Stage 2. The stage 1 record changes into one containing a mixture of sporadic bursts of 12-14 Hz. sleep spindles (primarily in the frontal regions) with high amplitude K-complexes. These wave forms are superimposed on a background of relatively low-voltage, mixed frequency EEG activity. Although it accounts for about half of total sleep time, this stage has attracted little research attention.
Stages 3 and 4. The EEG during these stages contains high amplitude, slow wave delta activity (1-3 Hz). The stages are differentiated primarily by the density of delta. Stages 2, 3, and 4 combined are sometimes called stage NREM.
Stage REM. This stage consists of relatively low-voltage, mixed frequency EEG activity, similar to that found in stage 1 and during aroused waking periods. Alpha activity is generally absent; if present, it is sparse, and usually 1-2 Hz. slower than waking frequency. Concurrently, sporadic bursts of conjugate rapid eye movements (REM) and a relative decrease in submental electromyogram (EMG) activity occur. Paradoxically, stage REM involves activated and irregular autonomic and physiological functions (such as penile erection, and irregular breathing and heart rate) in spite of the relaxed musculature and the general appearance of S.
EEG diagnosis of sleep: Some limitations. For the objective diagnosis of sleep, these EEG criteria, supplemented by eye movement activity in stage REM, have been emphasized, often at the expense of other reliable physiological changes.
In practice, the simple classification of stages is complicated by a variety of irregularities observed in many sleep records. While the NREM stages (2, 3, and 4) are sometimes difficult to differentiate, they are relatively easily distinguished from waking and stage 1. Delta waves, however, may occur in awake Ss under the influence of barbiturates, and irregularities such as these may add confusion to the interpretation of otherwise relatively straightforward studies (Beh & Barratt, 1965).
The EEG diagnosis of sleep stages is most accurate when made over intervals of several minutes. When it is necessary to evaluate on-line segments of a few seconds’ duration, particularly during stage REM, the task becomes difficult and arbitrary (O’Connell, Gustafson, Evans, Orne, & Shor, 1965). The occurrence of transient alpha is particularly perplexing in this context. Short bursts of alpha are not uncommon in stage REM, particularly with Ss who generate a great amount of alpha when awake. Such intermittent alpha does not necessarily indicate transient awakening. In the absence of other evidence, however, alpha during sleep is best considered as indicating consciousness or arousal. Certainly, when S awakens fully, alpha appears in the EEG. The meaning of intermittent alpha is particularly difficult to evaluate when attempts are made to distinguish between waking and sleep (and hypnosis) using EEG criteria alone, or when attempts are made to present stimuli to sleeping individuals.
The sleep cycle. As S falls asleep, alpha activity becomes desynchronized and intermittent. After a short time in stage 1, S typically passes through stages 2, 3, and 4, sometimes alternating between them for 90 minutes to 2 hours. Then stage REM emerges for the first time. This cycle of stages is repeated several times during the night. Stage 4 occurs almost exclusively in the first part of the night. Stage REM returns about every 90 minutes and becomes progressively longer (from a few minutes to over half an hour), dominating the second half of the night. Slightly less than a quarter of an average night’s sleep is spent in stage REM. However, if stage REM is prevented from occurring (because of shortened sleep, drugs, experimental awakenings, or other factors), time in stage REM will increase on subsequent nights until at least some of the lost stage REM is recovered.
Perhaps the most significant recent discovery in sleep research was reported by Aserinsky and Kleitman (1953). They observed that awakening S during a REM period almost always led to vivid reports of dreams, but waking S from NREM sleep did not usually lead to dream reports.2 The association between stage REM and dreaming stimulated considerable interest in sleep research. Several excellent reviews of the many sleep studies completed since 1953 are available (for example, Foulkes, 1966; Kales, 1969; and Oswald, 1962).