
- 178 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Quantum Mechanics, Second edition
About this book
Quantum mechanics is the key to modern physics and chemistry, yet it is notoriously difficult to understand. This book is designed to overcome that obstacle. Clear and concise, it provides an easily readable introduction intended for science undergraduates with no previous knowledge of quantum theory, leading them through to the advanced topics usually encountered at the final year level.Although the subject matter is standard, novel techniques have been employed that considerably simplify the technical presentation. The authors use their extensive experience of teaching and popularizing science to explain the many difficult, abstract points of the subject in easily comprehensible language. Helpful examples and thorough sets of exercises are also given to enable students to master the subject.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Preliminary concepts
This chapter presents the basic physical ideas that underpin the quantum theory. The central features are uncertainty and discreteness. The conceptual foundations of the theory involve some notorious subtleties, but these need not get in the way of progress. The reader may find it helpful to return to this chapter having mastered the later technical sections.
1.1 ORIGINS
Historians place the beginnings of the quantum theory at 1900. That year Max Planck published his famous formula for the distribution of energy as a function of frequency in black-body radiation. The details need not concern us. It was here that a new fundamental constant was introduced into physics – Planck’s constant h. More often we shall use h/2π, denoted by .
Although Planck did not fully grasp the significance of this step, with the benefit of hindsight the pertinent features are as follows. Electromagnetic energy, which prior to these developments was regarded as continuous, in fact comes in discrete lumps or packets – quanta – called, in this case, photons. The discrete nature of radiation manifests itself in the processes of absorption or emission by matter, which occurs only in multiple units of these energy packets. The energy is not the same for all photons but is related to the frequency v of the electromagnetic wave by
(1.1) |
or, equivalently, where ω = 2πv, a fundamental formula of quantum theory. Note carefully that the high-frequency (short-wavelength) photons are the most energetic. For light, v ≈ 1015 Hz, so E = 10−18 J – negligible in the macroscopic world. Only at the atomic level is equation (1.1) of importance.
A further important formula follows from the relation between energy E, momentum p, and rest mass m according to the theory of relativity:
(1.2) |
Classical electromagnetic theory connects E and p for an electromagnetic wave through the relation
(1.3) |
where p = |p|. Comparing equations (1.2) and (1.3) reveals that for a photon m = 0. For this reason the photon is sometimes called a ‘massless particle’. From equations (1.1) and (1.3) we then arrive at
(1.4) |
where λ is the wavelength and k the wave number.
Students sometimes ask why electromagnetic energy should manifest itself in packets. It is important to realize that this phenomenon cannot in some way be derived from classical electromagnetic theory. It must be accepted as a simple fact. That is the way nature is.
Curiously, although radiation theory gave birth to quantum mechanics, the interaction of matter and electromagnetic radiation is a rather advanced topic in quantum mechanics and it took almost fifty years for an acceptable formulation to be achieved. One reason for this is that photons are quanta of the electromagnetic field, and the quantum theory of fields requires a relativistic treatment to be entirely successful.
Confirmation of the photon hypothesis comes from the photoelectric effect. A stream of radiation, when impinging on a metal surface, can knock electrons out after the fashion of a coconut shy. Generally, one photon knocks out one electron. We see this by increasing the flux; more electrons come out because more photons are arriving. Similarly, increasing the frequency, the electrons emerge with greater energy, in accordance with equation (1.1). These features would be perplexing on the basis of the old classical theory of electromagnetism.
1.2 COLLAPSE OF DETERMINISM
The discrete character of electromagnetic energy leads directly to the need for a profound modification in our understanding of the physical world. Consider a polarized light wave which encounters a polarizer astride its direction of propagation. If the polarizer is oriented parallel to the direction of polarization, all the light is transmitted; if the polarizer is perpendicular to the direction of polarization, none is transmitted. At 45° half the light gets through. Generally the transmitted fraction is cos2 θ, where θ is the angle of the polarizer axis relative to the polarization plane (Fig. 1.1).
All this is readily comprehensible according to Maxwell’s electromagnetic theory, using vector projections; at 45° the polarizer takes out half the wave energy. Weird overtones develop, however, when the quantum nature of light is taken into account. Suppose the intensity of the light is reduced so that only one photon at a time arrives at the polarizer. Because photons cannot be chopped in half, each photon either does, or does not, get through the polarizer. That much is obvious. But there is nothing to distinguish any one photon from any other – the polarizer has no means of sorting them into ‘sheep’ and ‘goats’. If it sometimes passes a photon and sometimes blocks it, without rhyme or reason, all we can say is that there is a fifty-fifty chance of any given photon being passed. Thus, on average, half the photons get through and half do not. We may say that a particular photon will be transmitted through the polarizer with a probability of 0.5. As the orientation θ is varied, so the odds in favour of transmission vary as cos2 θ.
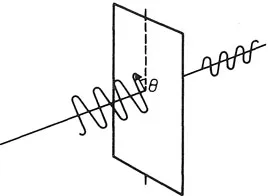
Fig. 1.1 When plane-polarized light encounters an obliquely oriented polarizer only a fraction cos2 θ of the intensity is transmitted, corresponding to that component of the polarization vector that lies along the polarization axis. In quantum language this implies that only a fraction of the incident photons is transmitted, each photon with probability cos2 θ.
The injection of this fundamental probabilistic element into physics is a major conce...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- 1 Preliminary concepts
- 2 Wave mechanics 1
- 3 One-dimensional delta wells
- 4 Wave mechanics 2
- 5 Scattering in three dimensions
- 6 The formal rules of quantum mechanics
- 7 Angular momentum
- 8 Particle in a central potential
- 9 Some atomic nhvsics
- 10 Approximation methods
- 11 Transitions
- Appendix A The one-dimensional infinite array of delta wells (Kronig-Penney model)
- Appendix B Spherical harmonics
- Appendix C The Dirac delta function
- Appendix D Useful quantities
- Appendix E Useful integrals
- Appendix F Exercises
- Appendix G Answers to exercises
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Quantum Mechanics, Second edition by Paul C.W. Davies,David S. Betts in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physics. We have over one million books available in our catalogue for you to explore.