
eBook - ePub
Tobacco-Specific N-Nitrosamines Recent Advances
Roger O. McClellan
This is a test
Share book
- 85 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Tobacco-Specific N-Nitrosamines Recent Advances
Roger O. McClellan
Book details
Book preview
Table of contents
Citations
About This Book
This remarkable book provides updates on various aspects of tobacco - its chemical and biological nature, and its physiological effects.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Tobacco-Specific N-Nitrosamines Recent Advances an online PDF/ePUB?
Yes, you can access Tobacco-Specific N-Nitrosamines Recent Advances by Roger O. McClellan in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.
Information
Article 7
COMPARATIVE CARCINOGENICITY, METABOLISM, MUTAGENICITY, AND DNA BINDING OF 7H-DIBENZO[c,g]CARHAZOLE AND DIBENZ[a,j]ACRIDINE
Comparative Carcinogenicity, Metabolism, Mutagenicity, and DNA Binding of 7H-Dibenzo[c,g]carhazole and Dibenz[a,j]acridine
Department of Environmental Health, University of Cincinnati, P.O. Box 670056, Cincinnati, OH 45267–0056
*To whom correspondence should be addressed.
ABSTRACT: Complex mixtures that are produced from the combustion of organic materials have been associated with increased cancer mortality. These mixtures contain homocyclic and heterocyclic polycyclic aromatic hydrocarbons (PAHs), many of which are known carcinogens. In particular, N-heterocyclic aromatic compounds (NHA) are present in these mixtures. Studies to determine the metabolic activation of these compounds have been undertaken. The purpose of this review is to compare and contrast the metabolic activation and biological effects of two NHA, 7H-dibenzo[c,g]carbazole (DBC) and dibenz[a,j]acridine (DBA), in order to better assess the contribution of NHA to the carcinogenic potency of complex mixtures and to develop biomarkers of the carcinogenic process. DBC has both local and systemic effects in the mouse; it is a potent skin and liver carcinogen following topical application and a lung carcinogen following i.p. application. On the other hand, DBA is a moderate mouse skin carcinogen following topical application and a lung carcinogen following subcutaneous injection. The biological differences for DBC and DBA are reflected in target organ-specific proximate and mutagenic metabolites and DNA adduct patterns.
KEY WORDS: 7H-dibenzo[c,g]carbazole dibenz [a,j] acridine N-heterocyclic aromatic compounds DNA binding metabolism carcinogenicity mutagenicity organotropism
I. INTRODUCTION
Exposure to complex mixtures resulting from combustion processes such as soot, coal tar and pitch, mineral oils, and coal gasification residues has been associated with increased cancer mortality.1, 2, 3, 4, 5, 6, 7 These mixtures contain polynuclear aromatic compounds that are released into the environment from sources ranging from smoke stack and coke oven effluents to tobacco smoke; the carcinogenicity of these mixtures is well recognized.1, 2, 3, 4, 5, 6, 7 Studies of the PAH fractions have been the primary focus of efforts to define the carcinogenicity of mixtures.2,3,4,8–10 Benzo[a]pyrene (BaP) has been the best-characterized PAH as a potential indicator of biological activity of the PAH fraction.2 NHAs have been identified in neutral and basic fractions of complex mixtures from motor vehicle exhaust,11 effluents from coal and oil processing,12 coke oven emissions,13 coal tar-based therapeutic agents,14 tobacco smoke condensates,15 carbon black emissions,16 synthetic fuels,17 sea water,18 industrial and urban atmospheres,19,20 and coal gasification residues.21 Based on the contribution of NHA to complex mixtures, we have attempted to study the metabolic activation of NHA. In particular, we selected DBC and DBA (Figure 1) as model compounds for our studies based on animal testing of members of that class of compounds, much of which was done between 1930 and 1950. Both DBC and DBA were found to be carcinogenic in more than one tissue. Additionally, both compounds were symmetric around their C2 axis, with the only difference being that the middle ring for DBC had a pyrrole ring whereas DBA had a pyridine ring. Therefore, it was hoped that the information obtained could be used to develop a structure- activity relationship for NHA. This review addresses what is known about DBC and DBA, and compares and contrasts the results obtained for their carcinogenicity, metabolism, mutagenicity, and DNA binding.
II. CARCINOGENICITY
NHAs have been studied for the past 60 years.1 DBC has been shown to be a potent carcinogen to mouse skin and mouse liver by topical application and subcutaneous injection as well as being a pulmonary and forestomach carcinogen in mouse following subcutaneous injection and oral administration, respectively.22, 23, 24, 25, 26, 27, 28, 29, 30 DBC was also shown to be carcinogenic in mouse lung following intravenous injection31 and in mouse liver following intraperitoneal administration.32 DBC was carcinogenic in the urinary bladder of the dog following intravesical injection33 and in the rat following subcutaneous injection.23 DBC was carcinogenic in hamster lung with and without carrier dust, ferric oxide,34, 35, 36 following intratracheal administration. Using doses comparable to BaP plus ferric oxide, DBC plus ferric oxide was more carcinogenic in the hamster lung.37 In summary, DBC was carcinogenic in mouse, rat, hamster, and dog, with both local and systemic effects (Table 1).
On the other hand, DBA was found to be a moderate to weakly active carcinogen to mouse skin by topical application and subcutaneous injection.38, 39, 40, 41, 42 DBA was also carcinogenic in mouse lung following subcutaneous injection.31 However, DBA was not active in the hamster and rat lung following either intratracheal administration or injection into the lung, respectively.43,44 Similarly, when DBA and ferric oxide were given together intratracheally to hamsters, no effect was seen44 (Table 2).
In order to begin our work on the metabolic activation of DBC and DBA, we felt it was necessary to assess DBC and DBA in the mouse skin model in a complete carcinogenesis protocol as well as in an initiation-promotion protocol. This reasoning was based on our evaluation of earlier studies that displayed several shortcomings: the purity of the compound was not always reported, benzene was used as a solvent in some cases, toxic doses were used in several instances, and an inadequate number of controls were used in many cases. Second, it was important to compare the carcinogenicity of these two compounds to each other as well as to a third, well-known carcinogen, BaP, because up to that point no such study had been undertaken.
The sources and purity of DBC, DBA, and BaP were reported.45 DBC was synthesized in the laboratory and purified by recrystallization to greater than 99% purity. Both DBA and BaP were purchased with 99% purity and were further purified to 99.5% purity by column chromatography in conjunction with recrystallization. The purity of all three compounds was monitored by high-performance liquid chromatography (HPLC), UV, and mass spectrometry.
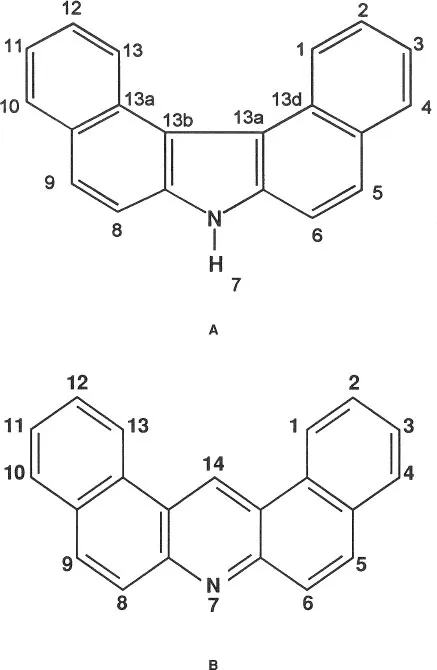
FIGURE 1. Structure of (A) 7H-dibenzo[c,g]carbazole (DBC) and (B) dibenz[a,j]acridine (DBA).
This laboratory has a long history of using mouse skin carcinogenicity studies to examine the mechanism of action of a variety of compounds and materials, such as PAHs, coal tar, asphalt, and various petroleum and shale oil products.2, 3, 4 The C3H mouse was the model of choice for the mixtures and compounds mentioned above. Thus, although the C3H mouse appeared to be less sensitive in complete carcinogenicity and in initiation-promotion studies, it was the animal of choice in our first study due the historical database.46 Based on the fact that solutions of 0.1 to 0.5% for DBC and DBA were toxic in prior studies, 50 male C3H mice per group were treated twice a week with 12.5 μg (0.025% solution) of BaP (49.6 nmol), DBC (46.8 nmol), or DBA (44.8 nmol) delivered in 50 μl of acetone to the interscapular region of the back. An acetone group of 50 mice received 50 μl of distilled acetone twice weekly. A no-treatment group of 50 animals was also included. Applications were continued for 99 weeks or until the animal became moribund. Both DBC and BaP produced 48 skin tumors in 50 mice, of which 47 in each group were squamous cell carcinomas and one papilloma, while DBA produced 25 tumors, of which 22 were squamous cell carcinomas and 3 papillomas. The latency periods for DBC, BaP, and DBA were 36.6, 32.4, and 80 weeks, respectively. Based on the latency and tumor incidence, DBC was found to be as potent a skin carcinogen as BaP and more so than DBA. No liver tumors were found for DBC, probably due to the rapid appearance of malignant skin tumors. No tumors were seen in the acetone or no-treatment groups.46
TABLE 1
Tumor Studies for DBC
Tumor Studies for DBC
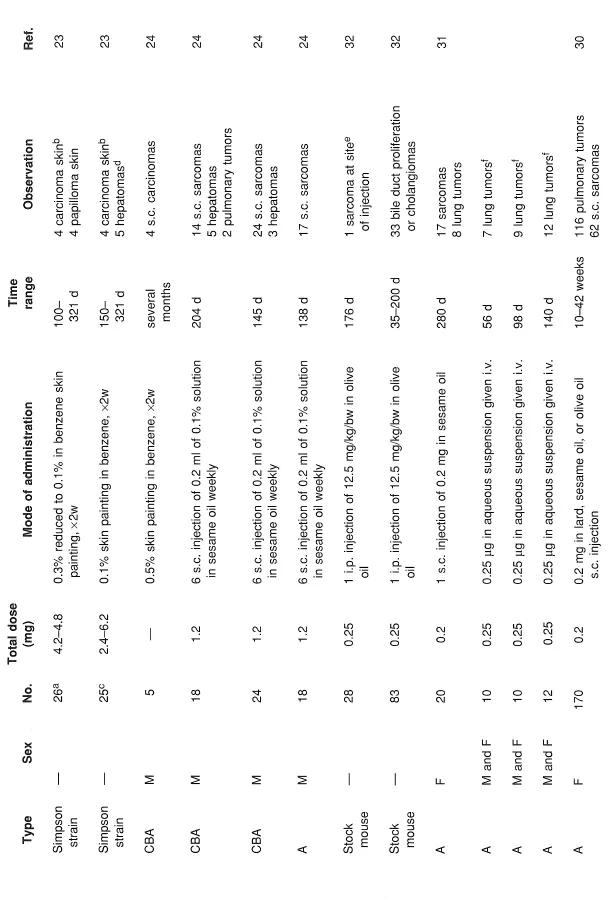
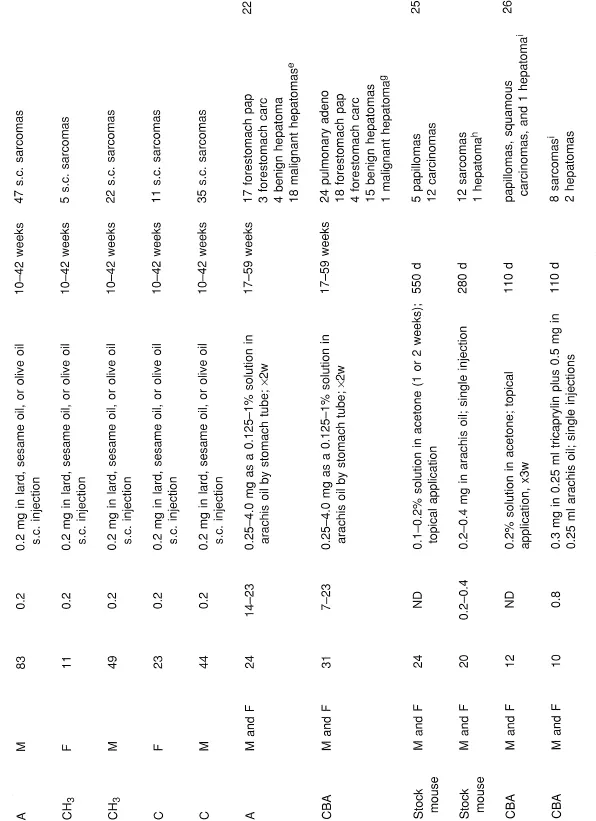
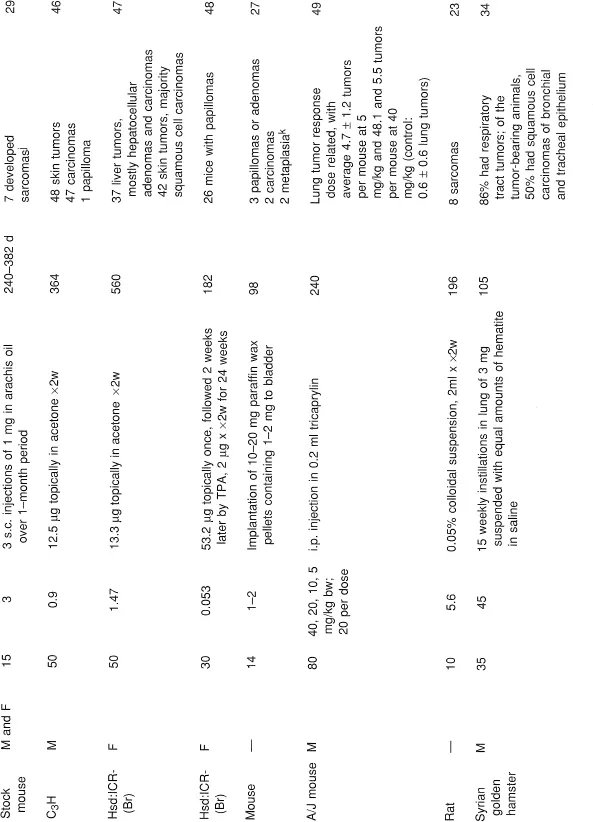

a Thirty-four died early.
b Eighty percent of those who survived a month showed hypertrophic biliary cha...