
- 338 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Physics for Animators
About this book
Achieving believable motion in animation requires an understanding of physics that most of us missed out on in art school. Although animators often break the laws of physics for comedic or dramatic effect, you need to know which laws you're breaking in order to make it work. And while large studios might be able to spend a lot of time and money testing different approaches or hiring a physics consultant, smaller studios and independent animators have no such luxury. This book takes the mystery out of physics tasks like character motion, light and shadow placement, explosions, ocean movement, and outer space scenes, making it easy to apply realistic physics to your work.
- Physics concepts are explained in animator's terms, relating concepts specifically to animation movement and appearance.
- Complex mathematical concepts are broken down into clear steps you can follow to solve animation problems quickly and effectively.
Uniting theory and practice, author Michele Bousquet teaches animators how to swiftly and efficiently create scientifically accurate scenes and fix problem spots, and how and when to break the laws of physics. Ideal for everything from classical 2D animation to advanced CG special effects, this book provides animators with solutions that are simple, quick, and powerful.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Section 1
Classical Physics
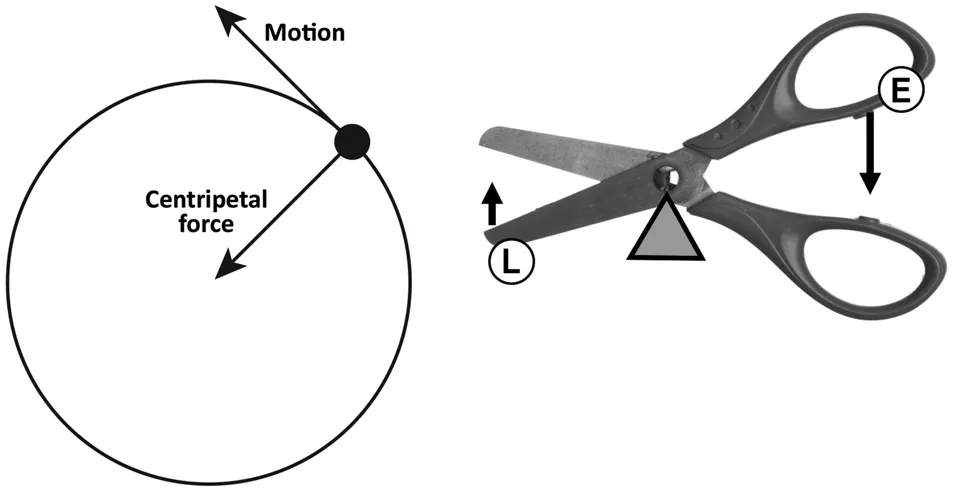
Chapter 1
Matter and Masses
Matter



Composition of Matter

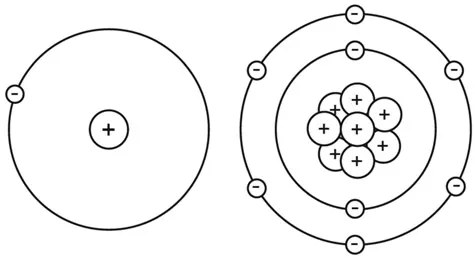
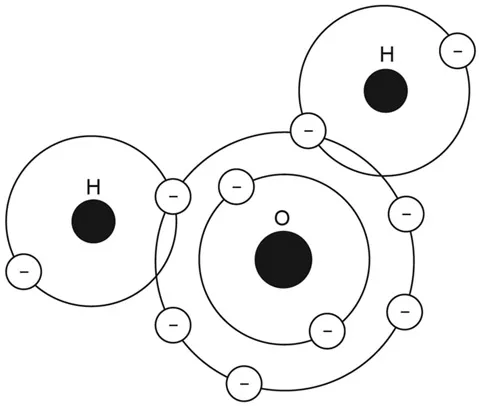
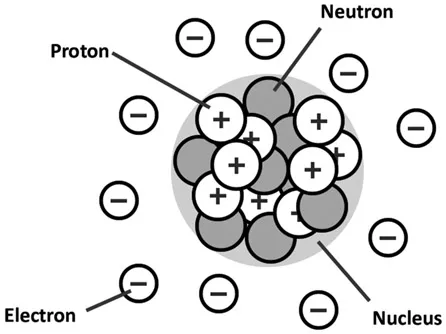
Atoms and Binding
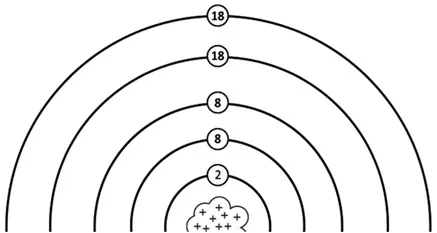

Table of contents
- Cover Page
- Half Title page
- Title Page
- Copyright Page
- Contents
- Acknowledgements
- Foreword
- Introduction
- 1 Classical Physics
- 2 Character Design and Animation
- 3 Visual Effects
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app