
- 286 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
The story of human evolution has been told hundreds of times, each time with a focus that seems most informative of the teller. No matter how it is told the primary characters are rarely mothers and infants. Darwin argued survival, but today we know that reproduction is what evolution is all about. Centering on this, Trevathan focuses on birth, which gives the study of human evolution a crucial new dimension.Unique among mammals, humans are bipedal. The evolution of bipedalism required fundamental changes in the pelvis and resulted in a narrow birth canal. Humans are also large-brained animals, which means that birth is much more challenging for our species than for most other animals. The result of this mismatch of large head and narrow pelvis is that women are highly dependent on assistance at birth and their babies are born in an unusually undeveloped state when the brain is still small. Human Birth discusses how the birth process has evolved and ways in which human birth differs from birth in all other mammals.Human Birth is also concerned with mother-infant interaction immediately after birth. While working as a midwife trainee, Trevathan carefully documented the births of more than one hundred women and recorded maternal and infant behaviors during the first hour after birth. She suggests ways in which the interactions served not only to enhance mother-infant bonding, but also to ensure survival in the evolutionary past. With clarity and compelling logic Trevathan argues that modern birth practices often fail to meet evolved needs of women and infants and suggests changes that could lead to better birth experiences. This paperback edition includes a new introduction by the author.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
MedicinaCHAPTER
1
EVOLUTIONARY PERSPECTIVES ON HUMAN BIRTH AND BONDING: THE BACKGROUND
All characteristics and behaviors of a species ultimately can be evaluated in terms of their reproductive consequences. Natural selection has favored and will continue to favor genetically based characters and behaviors that enhance reproductive success. The fact that fitness is measured in terms of reproductive success, however, does not necessarily mean that “more is better.” Just as in economics, there is always an upper limit, a point at which the costs of producing or acquiring more far outweigh the benefits of each unit of increase. Competing selection pressures operate on all organisms, and rarely are responses made without compromise. Compromise can be made at various levels. At the individual level a trait can be favored up to the point at which it becomes detrimental to the individual or extracts energy from development of another trait. Within a group, an individual’s fitness can be favored to the point at which each unit increase has a negative effect on other members of the group carrying that individual’s genes. The reason that selection has favored compromise solutions throughout the history of life is that fitness is measured in generational, not in individual, terms. In other words, how many offspring one individual produces is not so important as the number of that individual’s genes represented many generations later. In the long run, the amount of energy expended by an individual in the reproductive effort is far less important than the benefit to future fitness.
Although most of this book is concerned with human birth and mother-infant interaction immediately after birth, these two areas of concern are only a small part of the overall human reproductive strategy. In order to appreciate what goes on during birth and why mothers and infants behave the way they do after birth, it is important to have some understanding of the long series of steps that have been taken in the evolution of this reproductive strategy from the earliest sexually reproducing organisms to technologically managed births of today. The phylogenetic background of human birth and bonding is the subject of this chapter.
Sex
One of the first compromises reached in the evolution of the human reproductive strategy, beginning with very simple forms of life, was the compromise of sex. Explaining the origin of sexual reproduction is one of the greatest problems confronting evolutionary theory (Fisher, 1930; Maynard Smith, 1971; Williams, 1975). If the goal is to produce more, then the optimal strategy for an individual, it would seem, would be to reproduce asexually so that all of its genes are included in each offspring. Sexual reproduction entails the cost of meiosis (only one-half of an individual’s genes are passed on) and the cost of recombination (the risk of breaking up a good combination of alleles for the production of a potentially lethal combination). And yet, vertebrates are overwhelmingly committed to sexual reproduction.
The usual explanation offered is that sexual reproduction facilitates the production of new variability in organisms confronting frequent environmental challenges. For example, if a pathogen arises that is capable of destroying an entire population or species, only a mutation in an asexual species can afford possible immunity to the pathogen. In sexually reproducing forms, some individuals may have inherited a genetic combination that, by chance, confers resistance to the new pathogen. Most of the population may be wiped out, but individuals that can “utilize genetic variance generated by past natural selection” (Maynard Smith, 1971:166) will survive. In general, transgenerational changes afforded by recombination can proceed far faster in species reproducing sexually than they can in asexual organisms that are dependent on mutation for change. In other words, evolution can proceed faster in sexually reproducing organisms that it can in asexual organisms. In addition, as Müller (1964; cited in Daly and Wilson, 1983) pointed out, recombination allows the elimination of unfavorable mutations whereas all descendents of an asexual species are “stuck with” a deleterious mutation in a parental form. In this way, recombination allows avoidance of change which confers decrease in fitness.
In a similar vein, Bernstein and his colleagues (1985) propose that the origin of sexual reproduction can be found in the advantages gained in the repair of genetic damage (see Maynard Smith, 1971). DNA damage and mutation are constant challenges that can be met successfully if two alleles or chromosomes are inherited from both parents, at least one of which has a “normal” variant capable of masking the effects of the deleterious mutation. In other words, repair and masking are short-term benefits accruing to the individual who reproduces sexually, whereas variation is a long-term benefit accruing to future offspring. Since natural selection operates on the individual, immediate benefits better explain the origin of sex than variation, which is more appropriately seen as a consequence. In the long run, it is better for an organism to have 10 offspring, sharing one-half its genes, that in turn have 10 on down the line in perpetuity, than 1000 clones that become extinct through inability to survive an environmental perturbation.
The dimorphism associated with sex is so taken for granted by human beings that its significance can be easily overlooked. It is, in fact, important to the adaptive strategy of each species. Not only are the apparatus and mechanisms of reproduction different for the two sexes, but the roads to reproductive success are divergent, if not in outright conflict. Inasmuch as reproductive success, or the differential transmission of an individual’s genes to the next generation, is considered by evolutionists to guide an individual’s approach to its environment (through the effect of natural selection), these differences are indeed important.
Thus, another consequence of sexual reproduction is that, as Wilson (1975:314) notes, it is an antisocial force in evolution. This antisocial force is apparent not only between the sexes but also between parent and offspring. If parents and offspring are genetically identical, it is clear that any act on the part of the parent that enhances the survival of offspring would be favored by selection. In sexual reproduction an offspring and parent share only one-half their genes so that an act enhancing the survival of offspring increases, in subsequent generations, not only the parental genes but those of competitors, as well. With parents and offspring acting selfishly, conflict is inevitable. In some species, for example, it may be to the parents’ advantage to breed again as soon as possible and terminate care provided the most recent batch of young. The young, however, will inevitably do all that they can to retain parental attention (Trivers, 1974).
The antisocial force is evoked even more strongly in the relations between the sexes. The basis of sexual difference is dimorphism of and investment in the gametes. Females produce large, energetically expensive, immobile gametes, whereas males produce small, energetically inexpensive, mobile gametes. This disparity is exacerbated in mammals by the demands of pregnancy in females. Given these differences, it is adaptive for females to optimize each attempt at reproduction, whereas males are better served by maximizing fertilization (Williams, 1975). This has profound significance for our understanding of a species’ interaction with its environment, for the overall adaptive complex must represent a compromise between the optima for the two sexes.
In higher vertebrates, the female’s strategy of maximizing each attempt at reproduction may include expending time and energy in the protection and feeding of offspring. The more assistance she can obtain in this process, the greater the likelihood that more of her young will survive. This, of course, pits the sexes against each other at the outset; she improves survival of her genes if she convinces the male to remain following insemination. He, on the other hand, improves his chances by leaving as soon as impregnation of the female takes place. Obviously, some sort of compromise has been worked out between the sexes, and between the generations, or sexual reproduction would not be so common and pervasive today.
To some extent, the “choice” of mating and parenting strategies for the male (stay or leave; monogamy or polygamy) has something to do with the amount of postconception effort he can contribute to the production of offspring. In birds, both parents generally feed and care for the young. In most species, neither sex has an advantage over the other in caretaking abilities. Given equal ability in parenting, it is not surprising that approximately 90% of bird species mate monogamously, for a season or for life. Most of these species are also territorial, a behavior that further favors monogamy. In this case, a male has two options: He can provide care for the young, thus increasing the survival chances of his brood, or he can abandon the female after courtship and seek new mates. This latter choice often involves finding new territories, defending them against other males, and attracting females for breeding purposes, all of which are time- and energy-consuming activities. Thus, in many cases the benefits of remaining monogamous and providing care for the young outweigh the advantages of promiscuous mating, especially if, as seems likely, offspring survival rate is increased. In mammals, the male can do little beyond providing protection for the young, so it is more often the case that he abandons a female as soon as courtship and mating have been completed. Not surprisingly, monogamy is rare in mammals. The issue of paternal care of young will be pursued further in Chapter 3.
Viviparity
After sexual reproduction, the next adaptations of concern in the evolution of the human reproductive strategy are the steps of internal fertilization and internal gestation, or the evolution of viviparity from the primitive oviparous baseline. Each step resulted in a further reduction in numbers of offspring that can be reproduced with each generation. Species that release sperm and eggs for external fertilization can produce millions of gametes at a time with the only limitation being the production capacity of their ovaries and testes. In addition, there is little or no subsequent responsibility, once gamete release has occurred. If the female retains the eggs for internal fertilization, the numbers produced are limited not only by the capacity of her ovaries but also by the capacity of her body to hold the eggs until fertilization has occurred. The cost to both sexes is greater, but the zygotes receive an extra bit of protection during a critical period, so it is likely that a greater percentage of them survive than is the case with external fertilization. Thus, we have a second step in the trade-off of quantity for quality and a step in the direction of greater parental investment.
With internal gestation, the cost to the female becomes even higher and the resulting numbers of offspring produced even lower. Again, however, by affording greater protection to the young until they are fairly well developed, a female further increases the percentage of young that survive. Internal gestation also enables embryonic development to proceed in a homogeneous environment, relatively independent of temperature and humidity fluctuations in the external environment. Viviparity has apprently evolved independently in many animal lines, which suggests that it is a successful strategy. One estimate has it that viviparity evolved 75 times in reptiles, 22 times in fish (10 in Chondrichthyes, 12 in Osteoichthyes), 4 times in amphibians, and 1 time in mammals (Blackburn, 1981).
The steps involved in the evolution of viviparity are complex and will only be mentioned briefly here. In oviparous species, the corpus luteum is the only source of progesterone, the hormone responsible for inhibiting uterine contractions. When the corpus luteum dies, progesterone is withdrawn and uterine contractions begin, leading to oviposition. One key to viviparity is that the embryo must be retained in the uterus for a longer time, and progesterone production must continue so that contractions are inhibited until the fetus can survive outside of the mother’s body. In order to retain the embryo, temporary endocrine glands are required which serve to maintain gestation through successive stages of embryonic development. In some mammalian species, the corpus luteum persists throughout pregnancy. In others, including our own, its function as a secretor of progesterone is assumed by the placenta soon after implantation or in mid- to late pregnancy. In this case the corpus luteum usually disappears at the time the placenta becomes the primary endocrine organ maintaining pregnancy.
A developing embryo, whether retained or not, must be able to obtain nutrients and oxygen and must be able to excrete wastes and carbon dioxide. Nutrient provision can be assumed by the yolk (present, at least at certain stages, in both oviparous and viviparous animals), so the biggest challenge to evolving viviparity was gas exchange. Thus, the placenta likely evolved primarily as an organ for gas exchange, and only later, with large mammals, did it become an important agent of nutrient transfer.
The Hemochorial Placenta
We usually think of the placenta as one of the characteristics that distinguishes the eutherian mammals from other mammals. Indeed, the subclass is sometimes referred to as comprising the “placental mammals,” implying that others could be called “nonplacental.” Actually the placenta is an organ that probably evolved in conjunction with viviparity and thus evolved independently in several animal lineages. For this chapter, however, a review of its development in mammals will be sufficient.
When the fertilized mammalian egg, or zygote, reaches the blastocyst stage, two separate clusters of cells are distinguishable (Figure 1.1). One is the inner cell mass, which will develop into the embryo, amnion, and allantois. The second cluster, called the trophoblast, is the layer of cells lining the blastocyst, which will form the chorion and, ultimately, the placenta. The amnion, allantois, and chorion are often referred to as “extraembryonic membranes,” indicating that, although they arose from the same fertilized egg as the embryo, they are not part of the embryo and are shed at birth (Figure 1.2). The amnion serves to maintain the embryo in an aqueous environment, which is important for animals that lay their eggs or bear their young outside of the water. The allantois functions in the elimination of waste and the transmission of gases, fusing with the inner surface of the chorion to form the placenta. Part of the allantois forms the umbilical cord. The chorion encloses the amnion, embryo, yolk sac, and allantois and serves as an intermediary between the embryonic material and the surrounding environment. In eutherian mammals, the chorion is the membrane that is in direct contact with the inner lining of the uterus, or endometrium, and part of it, with the allantois and uterine tissues, forms the placenta.
FIGURE 1.1 Diagram of the mammalian blastocyst showing the trophoblast (outer rim) and the inner cell mass (at north pole). Reproduced from “Phylogeny of the Primates” by W. P. Luckett and F. S. Szalay. By permission of Plenum Publishing Company, New York. Copyright, 1975.
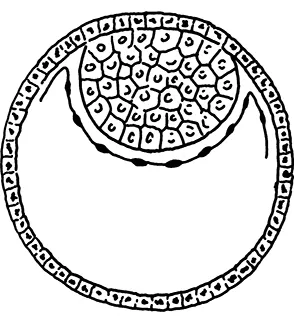
The most common placenta found in marsupials is the choriovitelline, a yolk sac-type of placenta in which the vascularized yolk sac fuses with the chorion. Marsupial embryos are surrounded by a shell membrane for most of the period of intrauterine development. When that membrane disappears near the end of pregnancy, placentation occurs, but for only a brief period before parturition takes place. Luckett (1975) proposes that the choriovitelline placenta is the ancestral placenta for the marsupials and eutherians, noting that a transitory form of this type is found early in pregnancy in a number of eutherians. The choriovitelline placenta is, for example, present in a transitory stage in primates such as lemurs and lorises, but it is absent in higher primates (for information on primate taxonomy, see Table 1.1). It should be noted that the chorioallantoic placenta characteristic of eutherian mammals is found in some marsupials, including bandicoots, of the family Peramelidae. Luckett (1975) suggests that this is an example of convergence in that chorioallantoic placentas occur in several lineages that have no immediate common ancestor.
The eutherian chorioallantoic placenta is one in which the chorion becomes vascularized by allantoic blood vessels. These placentas can be divided into several types, classified very generally according to their shape and structure, including the number of membranes between the maternal and fetal circulatory systems and, as mentioned previously, the degree of contact between the chorion and the uterine lining (Table 1.2). Two of concern in a survey of the Primate order are the epitheliochorial and hemochorial placentas. The former is found in swine, horses, donkeys, lemurs, and lorises, among others. The chorion is simply in close apposition to the uterine lining and the villi are widely diffused. There...
Table of contents
- Cover Page
- Title Page
- Copyright Page
- Table of Contents
- Introduction to the Paperback Edition
- Acknowledgments
- Introduction
- 1 EVOLUTIONARY PERSPECTIVES ON HUMAN BIRTH AND BONDING: THE BACKGROUND
- 2 ISSUES RELATING TO THE CURRENT STUDY: THE BIRTH CENTER, MIDWIVES, MOTHERS, AND METHODS
- 3 THE PROCESS OF PARTURITION
- 4 THE NEWBORN INFANT
- 5 MOTHER-INFANT INTERACTION IMMEDIATELY AFTER BIRTH
- 6 MOTHER-INFANT BONDING AT BIRTH
- 7 AN EVOLUTIONARY PERSPECTIVE ON HUMAN BIRTH AND BONDING: CONCLUSIONS
- Appendix A Translation of Spanish Dialogues
- Bibliography
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Human Birth by Wenda R. Trevathan in PDF and/or ePUB format, as well as other popular books in Medicina & Ginecología, obstetricia y partería. We have over one million books available in our catalogue for you to explore.