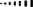![]()
1 | Molecular structure and bonding |
1.1 Isomerism
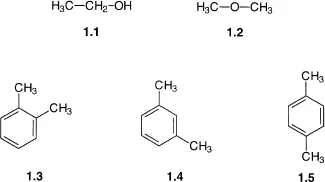
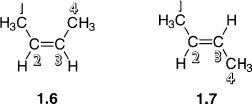
Isomers are different compounds that share the same molecular formula. Common examples include ethanol 1.1 and dimethyl ether 1.2, both of which have the molecular formula C2H6O; and 1,2-dimethylbenzene 1.3, 1,3-dimethylbenzene 1.4, and 1,4-dimethylbenzene 1.5, all of which share the molecular formula C8H10. In both of these cases, the connectivity of the atoms is different, i.e. in compound 1.1 the order in which the carbon and oxygen atoms are bonded is C–C–O, whilst in 1.2 the order is C–O–C. Because they differ in the connectivity of their atoms, compounds 1.1 and 1.2 are said to be constitutional isomers (also sometimes called structural isomers), as are compounds 1.3–1.5.
Constitutional isomers have different chemical and physical properties. This is markedly apparent in the case of isomers 1.1 and 1.2, since ethanol is a liquid at room temperature and undergoes all the chemical reactions that would be expected for a primary alcohol (dehydration to an alkene, oxidation to an aldehyde or acid etc.). Dimethyl ether 1.2 on the other hand is a gas at room temperature and is unaffected by the reaction conditions and reagents used to dehydrate or oxidize ethanol. This difference in reactivity is to be expected since the two compounds contain different functional groups.
Isomers 1.3–1.5 are more closely related since they contain the same functional groups (two methyl groups attached to a benzene ring), and differ only in the relative positions of these functional groups. Such isomers are often called positional isomers, but note that positional isomerism is a subclass of constitutional isomerism. Once again the three isomers have different physical properties, for example the boiling points are 144°C, 139°C and 138°C for 1.3, 1.4 and 1.5 respectively. In general, these three isomers will undergo the same types of chemical reaction (benzylic oxidation for example), but will do so at different rates, again illustrating their different chemical properties.
There is, however, another type of isomerism, one in which all of the atoms in the two isomers do have the same connectivity. A familiar example is found in 1,2-disubstituted alkenes such as compounds 1.6 and 1.7. In both of these isomeric compounds, the order in which the carbon atoms are joined together is C𝟙–C𝟚=C𝟛–C𝟜 and the only difference between them is that in isomer 1.6 the two methyl groups are on the same side of the double bond, whilst in isomer 1.7 the two methyl groups are on opposite sides of the double bond. Any pair of isomers which have the same connectivity of their atoms but which differ in the relative orientation of those atoms are called stereoisomers.
Stereoisomers are the topic of this book and the following chapters will investigate the different structural features which are responsible for stereoisomerism, and discuss the chemical, biological and physical consequences of the formation of stereoisomers. Both organic and inorganic compounds can exhibit stereoisomerism, and examples of each will be found throughout this book. Essentially, stereochemistry is concerned with the shapes of molecules, and the consequences of a molecule adopting a particular shape.
Later in this chapter, the way in which the shape of a molecule may be predicted using Valence Shell Electron Pair Repulsion Theory (VSEPR) will be introduced, and the nature of the bonding found in the most common chemical structures will be discussed. At the end of this chapter, the various classifications of stereoisomers will be introduced and these will be discussed in more detail throughout the remainder of this book. However, many of the structures seen later in this chapter are three dimensional, and before they are discussed it is necessary to understand the conventions used when representing three dimensional structures on a two dimensional piece of paper.
1.2 Drawing three dimensional chemical structures
Chemistry is dominated by two structures: the tetrahedron and the octahedron. A tetrahedral structure is found whenever a carbon atom is bound to four groups via four single bonds, whilst many transition metal complexes are octahedral. Since both of these structures are three dimensional they cannot easily be drawn on a two dimensional piece of paper. To overcome this problem, chemists have developed a number of conventions that will be introduced here.
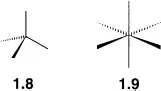
The usual way of drawing tetrahedral and octahedral structures is as shown in structures
1.8 and
1.9 respectively. In structure
1.8, two of the bonds are drawn with normal (−) symbols, and represent bonds in the plane of the paper. The other two bonds, however, are drawn with a wedge (
) and a hash (
) respectively. The wedge represents a bond in the direction drawn, which is also coming out of the paper towards you, whilst the hash represents a bond in the direction drawn and going into the paper away from you. The same symbols are used in structure
1.9 to illustrate the orientation of the bonds in an octahedral structure.
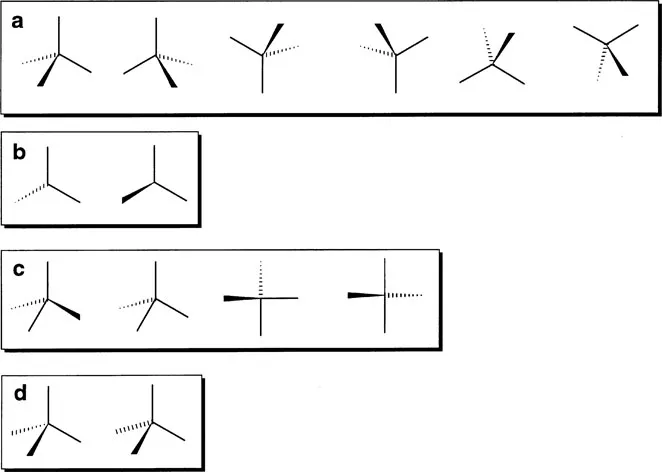
When used properly, this system of representing three dimensional structures with wedges and hashes provides a very simple and effective way of illustrating the structures of molecules. However, the correct use of the system requires some care. Structures 1.8 and 1.9 can be rotated to give a number of equivalent representations as shown in Figure 1.1a for a tetrahedral structure. It is very important, however, that a tetrahedral structure is drawn with the wedge and the hash on the same side of the structure, and that the bond angles are drawn to resemble the 109°@28' angles found in a perfect tetrahedron. A number of nonsense structures are shown in Figure 1.1c.
Figure 1.1 Correct (a and b) and incorrect (c and d) representations of a tetrahedron.
Another point to note when drawing structures with wedges and hashes is that the wedge and the hash are meant to convey the perspective of the structure; that is the wide end of the wedge/hash should be towards the reader. This is usually not a problem for wedges, but hashes are often drawn the wrong way round or with no change in width as illustrated in Figure 1.1d. Such structures will not be used in this text but they are common in the chemical ...