
eBook - ePub
Genetic Engineering Fundamentals
An Introduction to Principles and Applications
- 304 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This important reference/text provides technologists with the basic informationnecessary to interact scientifically with molecular biologists and get involved in scalinguplaboratory procedures and designing and constructing commercial plants.Requiring no previous training or experience in biology, Genetic EngineeringFundamentals explains the biological and chemical principles of recombinant DNAtechnology ... emphasizes techniques used to isolate and clone specific genes frombacteria, plants, and animals, and methods of scaling-up the formation of the geneproduct for commercial applications ... analyzes problems encountered in scaling-upthe microprocessing of biochemical procedures . .. includes an extensive glossary andnumerous illustrations ... identifies other resource materials in the field ... and more.Presenting the fundamentals of biochemistry and molecular biology to workers andstudents in other fields, this state-of-the-art reference/text is essentiai reading fortechnologists in chemistry and engineering; biomedical, chemical, electrical andelectronics, industrial, mechanical, manufacturing, design, plant, control, civil, genetic,and environmental engineers; chemists, botanists, and zoologists; and advancedundergraduate and graduate courses in engineering, biotechnology, and industrialmicrobiology.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Genetic Engineering Fundamentals by John Kammermeyer in PDF and/or ePUB format, as well as other popular books in Medicine & Biotechnology in Medicine. We have over one million books available in our catalogue for you to explore.
Information
1
Basic Components
Considering the complexity of a living organism one is struck by the relatively small number of organic components used in the formation of substances which control the functioning of the organism. For example, the basic control of the “building” of the organism is exercised by two chemicals: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). These materials consist of polymer chains containing monomer units called nucleotides, which are linked by phosphodiester bridges to form the linear polymer. The nucleotide, in turn, consists of three molecular components: a phosphate radical (Fig. 1), a ribose sugar (Fig. 1), and a nitrogen containing base (Fig. 2).
One may find that the structural formulas of bases are written in different forms by different authors. This is a result of their so-called tautomeric behavior. For instance, guanine can be written in its two forms as shown in the bottom line of Figure 2.
NOMENCLATURE OF COMPOUNDS
When the ribose-based complex is separated from the phosphate group the remaining structure is called a nucleoside. So, a phosphonucleoside is a nucleotide. Nucleotides may contain several phosphate residues, but are present in DNA and RNA only as monophosphates. The letter M in the compound name signifies monophosphate, D means di, and T is triphosphate. The conventional nomenclature is shown in Table 1. Because thymine was originally thought to occur only in DNA, the use of the term deoxythymine was considered to be redundant. Both TMP and dTMP are currently used.
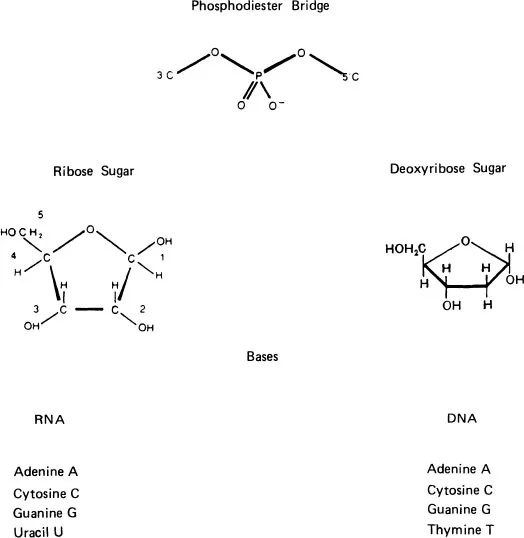
FIGURE 1 Formation of nucleotides. Basic components are tne phospho-diester bridge, ribose sugars, and bases. Combination rule for bases: Cytosine-C base pairs with Guanine-G; Adenine-A pairs with Uracil-U or Thymine-T (Hydrogen Bonding).
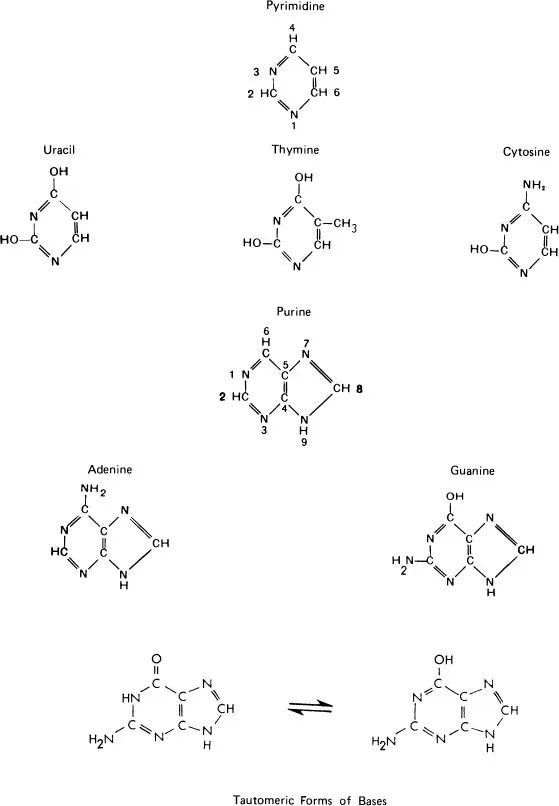
FIGURE 2 Basic compounds involved in DNA and RNA formation. The nitro-genous bases, pyrimidines, and purines.
TABLE 1 Conventional Nomenclature of Compounds
Base | Ribonucleoside | Ribonucleotide (5’-monophosphate) |
Adenine (A) | Adenosine | Adenylate (AMP) |
Guanine (G) | Guanosine | Guanylate (GMP) |
Uracil (U) | Uridine | Uridylate (UMP) |
Cytosine (C) | Cytidine | Cytidylate (CMP) |
Deoxyribonucleoside | Deoxyribonucleotide (5’-monophosphate) | |
Deoxyadenine (A) | Deoxyadenosine | Deoxyadenylate (dAMP) |
Deoxyguanine (G) | Deoxyguanosine | Deoxyganylate (dGMP) |
Deoxythymidine or Thymine (T) | Deoxythimidine or Thymidine | Deoxythymidylate or Thymidylate (dTMP) |
Deoxycytosine (C) | Deoxycytidine | Deoxycytidylate (dCMP) |
STRUCTURE
The single-stranded DNA polymer molecule associates with a second strand of complementary nucleotide sequence at physiological temperature, pH, and ionic strength. This is less likely to occur with RNA because usually there is no complementary RNA strand; however, it is very common for RNA to form doublestranded regions when complementary sequences occur within an RNA strand. Two DNA strands form a face-to-face configuration resulting in a double helix, and the strands are held together through hydrophobic base-stacking interaction between the adjacent planar bases (~80%) and hydrogen bonds between compatible bases (~20%).
Thus a very strict combining rule exists for the hydrogen bonding of pyrimidines and purines (1). A pyrimidine always bonds to a purine and vice versa, and does so in a very specific manner. That is, adenine (A) binds to thymine (T) or uracil (U), and cytosine (C) binds to guanine (G). There are two hydrogen bond locations in the A–T (or U) pairing and three hydrogen bond sites occur in the G–C complex. Note that hydrogen bonds occur only between noncarbon atoms of the base molecules. While this statement describes the opinion most generally held, the situation may change. The March 1, 1982 issue of Chemical and Engineering News (2) reports that the first verified hydrogen bond involving carbon atoms, that is–C–H–C, has been found in a ferrocene unit.
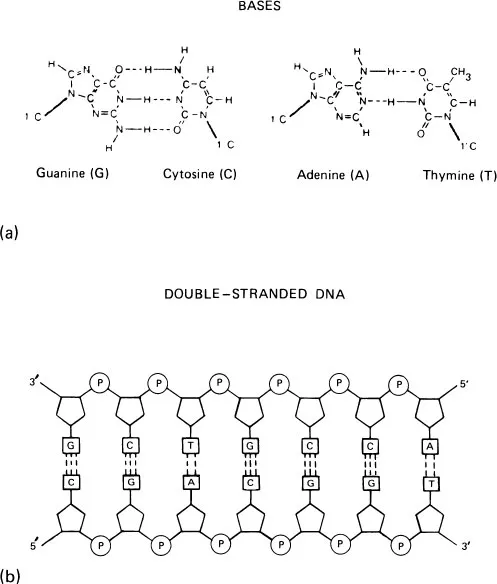
FIGURE 3 (a) Bonding of bases. Hydrogen bonding of G–C and A–T. (b) Hydrogen bonding in formation of double-stranded DNA.
A simple way to remember the pyrimidine-purine bonding is that alphabetically, the extreme letters A and T (or U) match up (and G–C, accordingly) and so pyrimidine bonds to purine. The A–T (or U) and G–C combinations are called base pairs. The hydrogen bonding and the polymer chain formation are illustrated in Figure 3.
The polymer chains of DNA and most RNAs differ in two aspects only:
DNA | RNA |
Deoxyribose | Ribose |
Thymine | Uracil |
(double-stranded) | (usually single-stranded) |
However, transfer and ribosomal RNA also contain a large number of “unusual” bases (3).
Attention should be called to the “numbering” convention. The bases (Fig. 2) are numbered in the usual system for organic compounds. To avoid confusion, the numbering of the ribose sugars (Fig. 1) is by a system of primes. This is shown in Figure 3, where the RNA has a 3’ and a 5’ terminal; so the 3’ bond in deoxyribose will combine with the 5’ terminal of the chain.
The 5’ and 3’ positions are of special importance, as they are the reaction sites involved in the mononucleotide addition to the growing DNA polym...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Introduction
- 1. Basic Components
- 2. The Cell
- 3. DNA, RNA, and Genes
- 4. Protein Synthesis
- 5. Enzymes
- 6. Plasmids, Viruses, and Microbial Hosts–Vectors
- 7. Recombinant Techniques
- 8. Nucleotide Sequencing and Hybridizing
- 9. Plant Genetics
- 10. Genetic Engineering Activities
- 11. Technology and Design
- 12. Techniques with Mammalian Cells
- 13. Precautions and Regulations
- 14. Update
- Appendices
- Index