
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Elements of Environmental Chemistry
About this book
A practical approach to environmental chemistry, Elements of Environmental Chemistry, 3rd Edition provides readers with the fundamentals of environmental chemistry and a toolbox for putting them into practice. This is a concise, accessible, and hands-on volume designed for students and professionals working in the chemical and environmental sciences.
The 3rd Edition has been completely revised and rearranged. The first chapter on tool skills has been expanded to include thermodynamic considerations and measurement issues. The former chapter on the partitioning of organic compounds has been expanded to cover the fates of organic compounds, with an emphasis on developing the reader's "chemical intuition" for predicting a chemical's fate based on structure. The material on lead, mercury, pesticides, PCBs, dioxins, and flame retardants has been expanded and combined into the last chapter and supplemented with more references to the literature. The problem sets have been extended and now include over 130 problems, some of which can be solved using Excel.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
Simple Tool Skills
1.1 Unit Conversions
| Prefix | Abbreviation | Multiplier |
| yocto | y | 10−24 |
| zepto | z | 10−21 |
| atto | a | 10−18 |
| femto | f | 10−15 |
| pico | p | 10−12 |
| nano | n | 10−9 |
| micro | μ | 10−6 |
| milli | m | 10−3 |
| centi | c | 10−2 |
| deci | d | 10−1 |
| kilo | k | 103 |
| mega | M | 106 |
| giga | G | 109 |
| tera | T | 1012 |
| peta | P | 1015 |
| exa | E | 1018 |

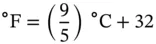
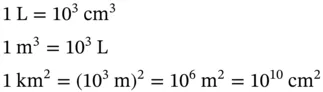
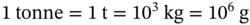
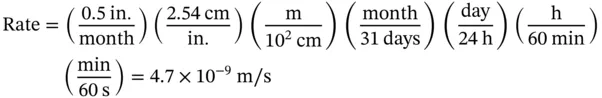
Table of contents
- Cover
- Table of Contents
- Preface
- 1 Simple Tool Skills
- 2 Mass Balance and Kinetics
- 3 Atmospheric Chemistry
- 4 Climate Change
- 5 Carbon Dioxide Equilibria
- 6 Fates of Organic Compounds
- 7 Toxic Stuff: Mercury, Lead, Pesticides, Polychlorinated Biphenyls, Dioxins, and Flame Retardants
- A Primer on Organic Structures and NamesPrimer on Organic Structures and Names
- B Periodic Table of the ElementsPeriodic Table of the Elements
- C Useful Physical and Chemical ConstantsUseful Physical and Chemical Constants
- D Answers to Problem SetsAnswers to Problem Sets
- Index
- End User License Agreement