
- 256 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Owen Bishop's First Course starts with the basics of electricity and component types, introducing students to practical work almost straight away. No prior knowledge of electronics is required. The approach is student-centred with self-test features to check understanding, including numerous activities suitable for practicals, homework and other assignments. Multiple choice questions are incorporated throughout the text in order to aid student learning. Key facts, formulae and definitions are highlighted to aid revision, and theory is backed up by numerous examples within the book. Each chapter ends with a set of problems that includes exam-style questions, for which numerical answers are provided at the end of the book. This text is ideal for a wide range of introductory courses in electronics, technology, physics and engineering. The coverage has been carefully matched to the latest UK syllabuses including GCSE Electronics, GCSE Design & Technology, Engineering GCSE and Edexcel's BTEC First in Engineering, resulting in a text that meets the needs of students on all Level 2 electronics units and courses. Owen Bishop's talent for introducing the world of electronics has long been a proven fact with his textbooks, professional introductions and popular circuit construction guides being chosen by thousands of students, lecturers and electronics enthusiasts.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part 1
Electricity
Topic 1
Electrons

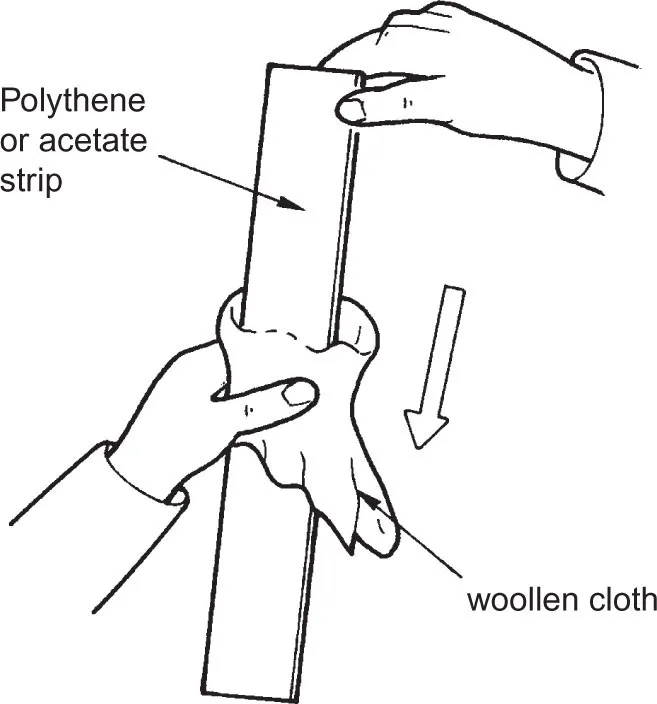

Things to do
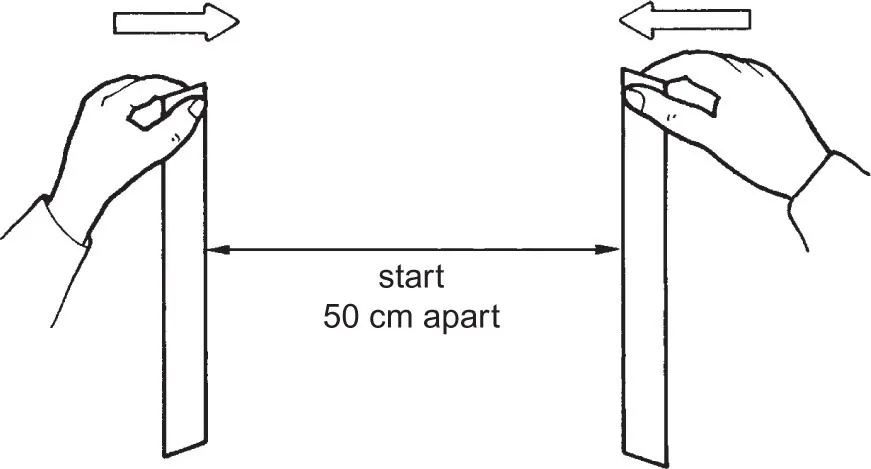
Self Test
KINDS OF CHARGE
Self Test
POSITIVE AND NEGATIVE CHARGE
USING ENERGY
ELECTRONS
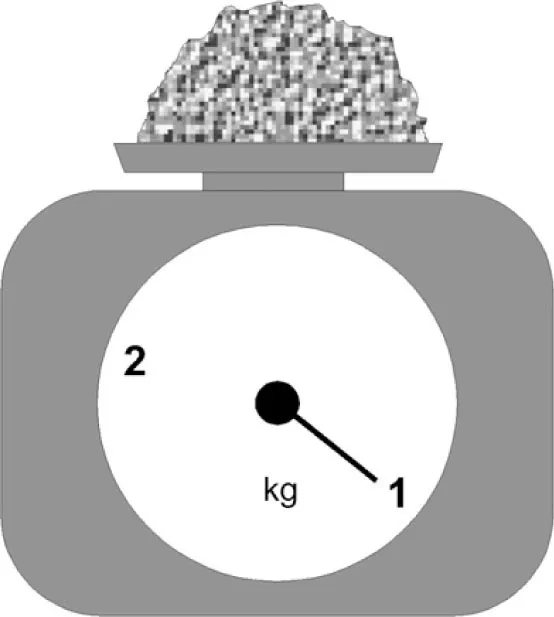
Self Test
ELECTRONS AND ATOMS

OTHER KINDS OF ATOM
Table of contents
- Cover
- Halftitle
- Title
- Copyright
- Contents
- Introduction
- PART 1. Electricity
- PART 2. Electronic Components
- PART 3. Electronic Systems
- PART 4. Electronic Systems in Action
- Supplements
- Acknowledgements
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app