
- 384 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Cell Biology of Trauma
About this book
This unique book presents an approach to viewing trauma. It examines the cellular consequences of trauma at a molecular level and provides new insights into the treatment of traumatic injury, based on cellular responses. The current of trauma research is reviewed, previously unpublished information on the topic is presented, and research directions are included.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Cell Biology of Trauma by John J. Lemasters,Constance Oliver in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biology. We have over one million books available in our catalogue for you to explore.
Information
Stress Response and Wound Healing
chapter 12
THE STRESS RESPONSE AND STRESS PROTEINS
Martin E. Feder, Dawn A. Parsell, and Susan L. Lindquist
TABLE OF CONTENTS
I. | Introduction | ||
II. | Inducible Stress Tolerance and Its Relationship to Stress Proteins | ||
III. | How Stress Proteins Function As Molecular Chaperones | ||
IV. | Regulation Of The Stress Response | ||
V. | Engineering Stress Tolerance | ||
Acknowledgements | |||
References | |||
I. INTRODUCTION
The stress response, or heat shock response, is a fundamental molecular mechanism with which organisms compensate for environmentally induced perturbations of cellular function. Heat and a variety of other stresses induce a suite of stress or heat shock proteins. These proteins play diverse cellular roles in minimizing or repairing damage due to stress, or in inducing tolerance to subsequent stress. Even in the absence of stress, these proteins or their close relatives are essential for a variety of cellular processes. Due to the vital nature of stress protein function and the importance of their induction as a model for gene regulation, interest in the stress response has undergone explosive growth, averaging more than 800 publications in the primary literature per year during the past five years. More than 500 publications examine the relationships between the stress response and the other subjects in this volume (e.g., reactive oxygen species, ischemia-reperfusion injury, inflammation). Accordingly, here we can but touch upon a limited number of aspects of the stress response that are relevant to the cell biology of trauma, and so can provide only a general introduction to the field. We will, however, cite many of the excellent recent reviews that provide entree to the primary literature.
II. INDUCIBLE STRESS TOLERANCE AND ITS RELATIONSHIP TO STRESS PROTEINS
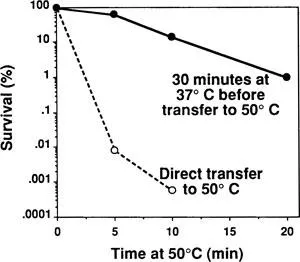
The general paradigm for inducible stress tolerance is this: If an unstressed cell or organism undergoes direct exposure to an extreme stress, cellular damage, if not death, will ensue. If, by contrast, the cell or organism first experiences a mild stress, it will become more tolerant of the extreme stress. Figure 1 exemplifies this phenomenon with data for yeast (Saccharomyces cerevisiae) cultured at 25°C and then either transferred directly to a high temperature (50°C) or pretreated at a mildly elevated temperature (37°C) for 30 min before exposure to 50°C; pretreatment improves survival at 50°C more than 1000-fold.1 Similar data are available for a wide variety of cell types, species, and stresses.2–4
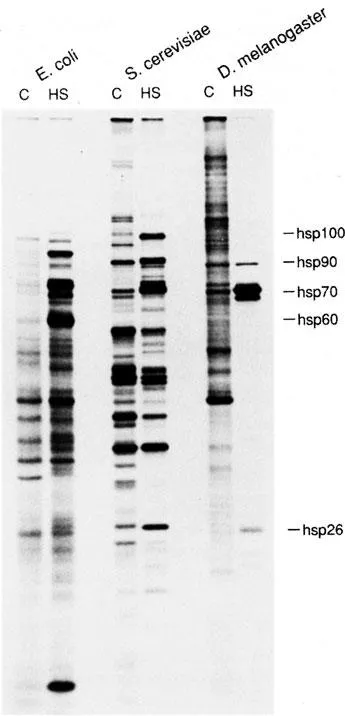
The mechanistic explanation of inducible thermotolerance began with the observation that exposure to heat induces puffing in the polytene chromosomes of Drosophila.5 Subsequent work established that the chromosomal puffing was a visible manifestation of the transcriptional activation of a characteristic suite of genes encoding proteins, originally termed heat shock proteins (hsps) (Figure 2). Several points are now evident:
• Hyperthermia is only one of many stresses that induce this same set of proteins. Known inducers of just one exemplary protein, hsp70, include high temperature, low oxygen (ischemia, hypoxia), ethanol, pH changes, H2O2 and other strong oxidants, free radicals, infection and inflammation, heavy metals, various toxins, ionizing radiation, extreme osmolar-ity, extreme levels of metabolites and normal intracellular regulators, cold, hemodynamic stress, and errors in protein synthesis, among others. For this reason, the terms stress protein and stress response are now in common use.
• Induction of stress proteins is a nearly universal feature of cells, and has been observed in every species in which it has been sought6 save one.7 Commonly, the induction occurs at environmentally appropriate levels of stress (e.g., the minimum temperature for stress protein induction is lower in fishes and insects from cold environments than in related organisms from hot environments).
• The major stress proteins can be assigned to at least five families or varieties of relatively large proteins (hsp100, hsp90, hsp70, hsp60, and Lon), and several more for smaller proteins (hsp27, DnaJ, GrpE, hspl0, and ubiquitin). Members of a family can be extraordinarily highly conserved. The human hsp70, for example, is 73% identical to the Drosophila protein and 50% identical to the Escherichia coli protein.
• Despite the ubiquity of the stress response and the conserved nature of stress proteins, organisms may vary considerably in their relative representation of particular stress proteins (Figure 2). In yeast, for example, hspl04 is one of the most prominent proteins induced by heat shock, whereas in Drosophila, expression of hsp70 predominates and a hsp100 family member is not evident. Members of the small hsp families, encoded by 30 to 50 different genes, dominate the response in many plants, whereas yeast and mammals synthesize only one small hsp.
The stress response initially attracted considerably attention as a model system for investigating gene structure and regulation. Exploitation of this model was highly successful: The genes for the Drosophila stress proteins were among the first eukaryotic genes to be cloned, to have their organization within the genome defined, to have their chromatin structure determined before and after activation, to have their regulatory sequences identified, and to have the transcription factors interacting with these sequences characterized. In addition, the stress response provided one of the first examples of selective gene expression operating at the level of translation. For a time, progress along these lines far outstripped efforts to understand the functions of the stress proteins themselves and the relationship of these functions to inducible stress tolerance. Since the mid-1980s, however, the application of molecular biological techniques and the emergence of novel hypotheses have set the stage for equal if not greater revelations concerning the function of stress proteins.
First, experimental studies have now demonstrated that stress proteins are not just correlated with inducible thermotolerance, but are essential for it. Deletion of the HSP104 gene in yeast, for example, results in a marked reduction in inducible thermotolerance, and introduction of the missing HSP104 gene on a plasmid restores inducible thermotolerance1 (Figure 3). Similar results have been obtained for hsp70 by varying cellular hsp70 levels either directly8,9 or through experimental manipulations of the corresponding genes.10, 11, 12, 13, 14 Moreover, stress proteins have been shown to confer tolerance to many different forms of stress. Hsp104, for example, improves tolerance to ethanol and arsenite in yeast and is expressed constitutively in spores, the most eurytolerant stage of the yeast l...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Preface
- The Editors
- Contributors
- Table of Contents
- Mechanisms of Lethal Cell Injury
- Cytoprotective Strategies
- Ischemia/Reperfusion Injury
- Stress Response and Wound Healing
- Preservation of Cells and Organs
- Index