
- 160 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Antibody Technology
About this book
This book provides a comprehensive overview of antibody technology. It discusses in detail the new generation of engineered antibodies and the latest developments in immunoassay techniques and applications, as well as describing conventional methods of antibody production and use. Antibody Technology will bring the reader up-to-date with current methods, helping the reader to make informed decisions on the best approach to a given task with regard to cost, time and final application.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
MedicineSubtopic
Immunology1 The Immune System
1.1 Introduction
The various systems of the mammalian organism are potentially at risk of infiltration by a wide variety of infectious micro-organisms. Most often, pathological damage is limited by the immune system. The immune system has two ways of overcoming such a challenge. The first of these arises from the innate immune system and the second from the adaptive immune system. The former is essentially a nonspecific first line of defence whereas the latter, as the name implies, is a mechanism specific to the immunological challenge. A further feature of the latter is a ‘memory’ effect which enables the immune system to react more rapidly and strongly should subsequent challenges be made by a given infectious agent.
The innate immune system comprises a variety of cells and humoral factors. The cells, namely phagocytes and natural killer (NK) cells, engulf and destroy ‘foreign’ particles. NK cells are also capable of binding to, and sacrificing, host cells that have exhibited cell surface changes as a result of viral infection or neoplasia. The actions of these cells are often mediated by soluble factors such as acute phase proteins including those of the complement cascade.
In certain situations, the invading micro-organism may not be effectively neutralized by the innate immune system, possibly because the micro-organism is not bound by the phagocyte either directly or via complement. In such circumstances, the mechanism of adaptive immunity is required to neutralize and eliminate the invading microbe. One requirement of this mechanism is the production of specific humoral factors capable of assisting phagocyte action which otherwise would not exist as part of the innate immune system. Such humoral factors are known as antibodies and are molecules produced by the B lymphocytes. Antibodies are the most important components of the immune system in the present context and are described in detail in Section 1.2.
B lymphocytes carry receptors on their surface capable of binding to surface molecules on the micro-organism. These receptors are similar in structure to the antibodies, which are ultimately secreted by the mature plasma cells derived from the B lymphocytes, in that they are glycoproteins known as immunoglobulins. The immune system possesses an enormous diversity of immunoglobulins capable of binding any large, ‘foreign’ invading molecule. Molecules capable of stimulating the immune response are known as antigens. The enormous number of possible antigens thus warrants great immunoglobulin (antibody) diversity.
1.2 Antibodies
Antibodies are glycoprotein molecules known as immunoglobulins. They are secreted by plasma cells derived from B lymphocytes which have been stimulated by binding of ‘foreign’ molecules to B-lymphocyte antigen receptors. The antibody has the same binding specificity as the antigen receptor.
Five classes of immunoglobulins exist in mammals and the characteristics of these are given in Table 1.1. The basic immunoglobulin unit consists of two heavy and two light polypeptide chains which are joined by disulfide bridges. The chains themselves also contain intra-chain disulfide bridges. Schematic structures of the basic immunoglobulin molecule are shown in Figure 1.1.
Each class of antibody possesses heavy chains characteristic of that class. Various subclasses show slight variation in the heavy chain structure within a given class. In all classes and subclasses the light chains can be one of two variants known as lc and X.
TABLE 1.1 Classes of antibody molecules
| Immunoglobulin | Molecular weight | Approx. plasma concn (ng ml−1) |
| IgG1 | 146 000 | 9 |
| IgG2 | 146 000 | 3 |
| IgG3 | 170 000 | 1 |
| IgG4 | 146 000 | 0.5 |
| IgM | 970 000 | 1.5 |
| IgA1 | 160 000 | 3 |
| IgA2 | 160 000 | 0.5 |
| IgA (secretory) | 385 000 | 0.05 |
| IgD | 184 000 | 0.03 |
| IgE | 188 000 | 5 × 10−5 |
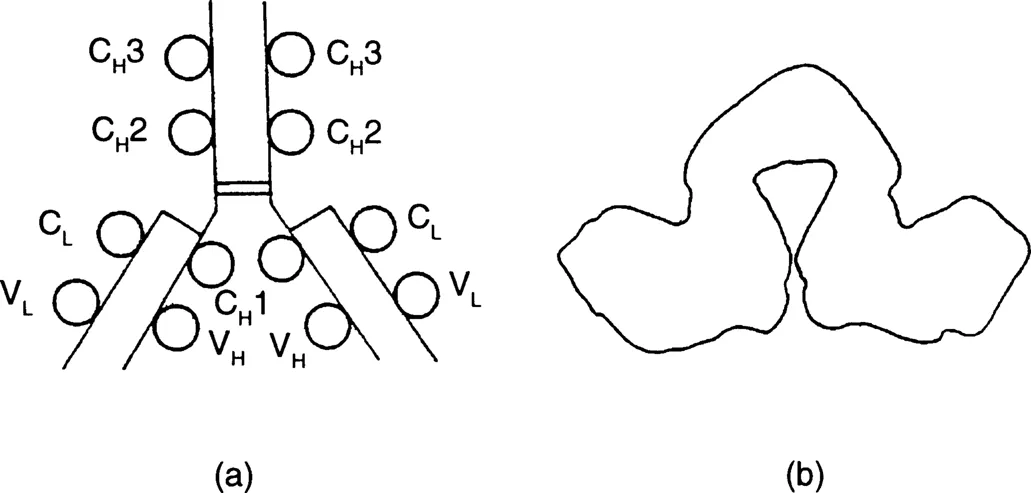
FIGURE 1.1 Schematic representation of a typical IgG molecule showing (a) structure and (b) shape. C, constant; V, variable; H, heavy; L, light. Polypeptide loops are formed by disulfide bridges to yield the various domains represented here as circles. The two heavy chains are joined by disulfide bridges. The light chains are also joined to the heavy chains by disulfide bridges. Carbohydrate chains are not shown here.
The molecules can be described as possessing both variable and constant regions, the latter being reasonably similar structurally within all antibodies within that class. The variable regions contain hypervariable regions which are responsible for antigen binding. The area of the hypervariable region which represents the actual binding site is known as the paratope and it is this region of the molecule which binds to the relevant antigenic determinant (epitope) on the antigen.
The different subclasses of immunoglobulin (Ig) are characterized particularly by differences in the number and position of inter-chain disulfide bridges. Immunoglobulin G (IgG) is the major immuno-globulin class in normal serum and is the major antibody of secondary immune responses, i.e. following a challenge subsequent to initial antigen exposure.
Immunoglobulin M (IgM) accounts for approximately 10% of the immunoglobulin pool, but is the major antibody of the primary immune response. It corresponds roughly to a pentameric form of the basic IgG molecule together with a so-called J (joining)-chain.
Immunoglobulin A (IgA) exists in circulating and secretory forms. In most mammals apart from man serum IgA exists predominantly as a dimer. IgA has two subclasses. Secretory IgA is dimeric consisting of two basic immunoglobulin molecules together with a J-chain and also a secretory component. IgA is the predominant immunoglobulin in seromucous secretions.
Immunoglobulin D (IgD) represents less than 1% of total immuno-globulins and its specific role remains unclear. It is however known to be present in relatively large amounts on the surface of circulating B lymphocytes.
Immunoglobulin E (IgE) is another trace circulating immunoglobulin but is found on the surface of basophils and mast cells. It is associated with acute hypersensitivity reactions such as allergies.
1.3 Antibody biosynthesis
It is not possible for each particular antibody specificity to be derived from a dedicated gene since there is simply not enough space in the genome to code for every conceivable antibody. Antibody diversity is thus generated in a number of other ways:
(i) multiple germ line variable region genes;
(ii) recombination of variable region gene segments with other gene sequences;
(iii) inaccuracy of recombination;
(iv) somatic mutation;
(v) combination of different heavy and light chains.
As can be seen from Figure 1.1, each immunoglobulin molecule possesses at least two variable regions, depending on the class of immunoglobulin. These variable regions in turn contain the paratope (i.e. that portion of the variable domain which binds to the epitope on the antigen). Both the light and heavy chains contain variable regions. The light chain also contains a constant domain whereas the heavy chain contains three constant domains.
Separate segments of DNA code for the variable (V) and constant (C) regions. In antibody-producing cells these segments are brought closer together. A further segment of DNA (J segment) is joined on to the V gene segment (Figure 1.2). A similar situation is seen in the case of heavy chains where a further gene segment (D segment) is joined on to the V and J segment genes (Figure 1.2). Thus diversity is generated by these recombinations and also by the fact that the position at which the various V, J and D segments combine may vary. In addition, it is common for further variation to be introduced due to point mutations in the V segment genes. Finally, additional diversity is generated due to the fact that almost any light chain may combine with any heavy chain.
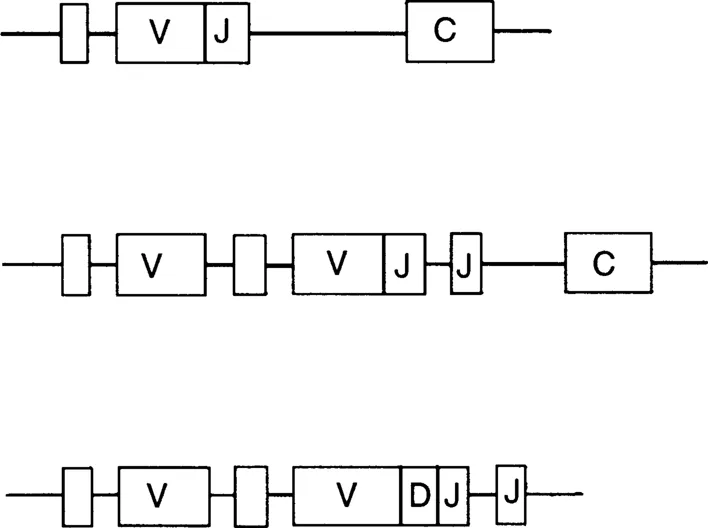
FIGURE 1.2 Schematic representation of examples of B-lymphocyte DNA coding for antibody domains. (Top) λ light chain, (middle) к light chain, (bottom)heavy chain variable domains. The sequences can be assembled as recombinations of many different V, C, J and D gene segments. Note that unlabeled boxes represent leader sequences.
Immunoglobulin class and subclass is described by the heavy chain constant region. All the constant region genes are positioned downstream from the J segment genes. During class switching as part of an immune response, the variable region specificity is maintained. Thus, the initial IgM antibodies and subsequent IgG antibodies will have the same specificity but will merely be different isotypes. This relatively minor diversity is known as isotypic variation and is in contrast to the wide diversity of the binding site ...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Abbreviations
- Preface
- Part 1: Basic Principles and Methods
- Part 2: Techniques and Applications
- Appendix A
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Antibody Technology by Eryl Liddell,Ian Weeks in PDF and/or ePUB format, as well as other popular books in Medicine & Immunology. We have over one million books available in our catalogue for you to explore.