
- 208 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Carbocation chemistry is not only fundamental to the advancement of organic chemistry, it also has found widespread applications in organic synthesis. It is not an exaggeration to say that carbocation chemistry is part of the foundation of organic chemistry. Carbocation Chemistry: Applications in Organic Synthesis provides a panoramic view of carbocation chemistry with an emphasis on synthetic applications.
This book is an invaluable tool for organic, medicinal and analytical chemists, including those working in biochemistry as well as the petroleum, plastics and pharmaceutical industries. It is also suitable for upper level undergraduates and graduates in organic chemistry, biochemistry and medicinal chemistry.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Carbocation Chemistry by Jie Jack Li in PDF and/or ePUB format, as well as other popular books in Medicine & Biochemistry in Medicine. We have over one million books available in our catalogue for you to explore.
Information
1 Introduction
CONTENTS
1.1 Nomenclature, Structure, and Stability
1.2 Generation of Carbocations
1.3 The Nonclassical Ion Controversy
1.4 Electrophilic Addition to Alkenes
1.4.1 Addition of Hydrohalides and Acetic Acid
1.4.2 Hydration
1.4.3 Addition of Halogens
1.4.4 Hydroboration of Olefins
1.4.5 Oxymercuration of Olefins
1.5 Electrophilic Aromatic Substitution
1.5.1 Mechanism and Orientation
1.5.2 Nitration
1.5.3 Halogenation
1.5.4 Friedel–Crafts Alkylation
1.5.5 Friedel–Crafts Acylation
1.6 β-Elimination Reactions
1.7 Rearrangement Reactions of Carbocations
1.7.1 Pinacol Rearrangement
1.7.2 Beckmann Rearrangement
1.7.3 Demjanov and Tiffeneau–Demjanov Rearrangement
1.7.4 Meyer–Schuster Rearrangement
1.7.5 Schmidt Reactions
1.7.6 Wagner–Meerwein Rearrangement
References
1.1 NOMENCLATURE, STRUCTURE, AND STABILITY
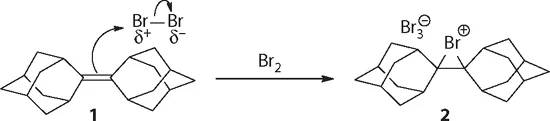
In sophomore organic chemistry, we learned that many organic compounds bear positive charges. For instance, bromonium ion was invoked as the putative intermediate when explaining the mechanism of electrophilic addition of bromine to alkenes. In 1985, Brown at Alberta isolated and determined the crystal structure of the bromonium ion 2 during the course of electrophilic bromination of adamantylideneadamantane (1).1
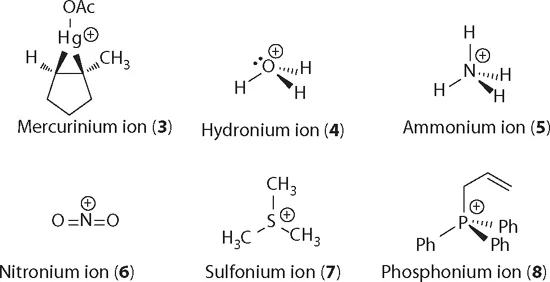
Other positive species in organic chemistry include mercurinium ion (3), hydronium ion (4), ammonium ion (5), nitronium ion (6), sulfonium ion (7), and phosphonium ion (8), just to name a few.
But none of the aforementioned -nium ions are as prevalent as carbonium ions. After all, organic chemistry is the study of carbon-containing compounds. As time passes, the term carbonium ions have been largely replaced by the more popular term, carbocations. This was probably inevitable since carbocation is one word and carbonium ion are two!
As a matter of fact, initially proposed by Olah2 and later accepted by the IUPAC (International Union of Pure and Applied Chemistry), the term carbocation encompasses two species, one is the carbenium ion, which represents the “classical” trivalent ions with CH3⊕ as the parent, and the other is the carbonium ion, which represents the “nonclassical” penta- (or higher) coordinate ions with CH5⊕ as the parent.
This book titled Carbocation Chemistry will cover both carbonium ions and carbenium ions. And in this chapter, carbocation and carbenium ion are used interchangeably because nearly all of the carbocations mentioned here are carbenium ions.
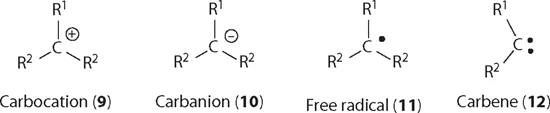
Carbocations depicted below as 9, with a cation on the carbon atom, is one of the important reaction intermediates in organic chemistry. The other intermediates include carbanions (10), radicals (11), carbenes (12), etc. For a trivalent carbocation, it is sp2 hybridized with a geometry of trigonal planar and all three bond angles are 120°.
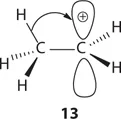
The stability of a carbocation is determined by its structure: the more alkyl substituents, the more stable. For tertiary (3°), secondary (2°), primary (1°), and methyl carbocations, their corresponding stability may be explained using the concept of hyperconjugation, which refers to the donation of a pair of bonding electrons into an unfilled or partially filled orbital. As depicted by ethyl cation (13), the two bonding electrons of the C–H σ-bond adjacent to the carbocation may donate part of its electron cloud to the carbocation's empty p orbital. As a consequence, cation 13 is more stabilized than it would if the α C–H bond did not exist. In all, ethyl cation 13 has three α C–H bonds, thus three hyperconjugations (a short hand to describe three possibilities of having hyperconjugation).
As clearly depicted below, t-butyl cation (14) as a representative of tertiary (3°) carbocations possesses nine α C–H bonds, therefore it has nine hyperconjugations. Meanwhile, isopropyl cation (15) as a representative of secondary (2°) carbocations possesses six α C–H bonds, therefore it has six hyperconjugations. In the same vein, primary (1°) cation 16 has three hyperconjugations and the methyl cation (17) has none. We can thus conclude that tertiary (3°) carbocations are the most stable in this series, followed by secondary (2°), primary (1°), and methyl carbocations. The stability of cations decreases in this sequence from the left to the right. This trend is, not surprisingly, consistent with experimental data generated from their dissociation energies in the gas phase.3
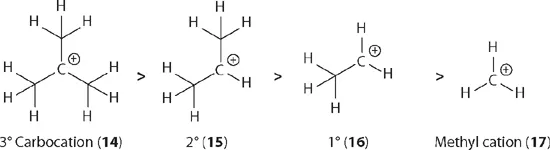
In addition to evoking the concept of hyperconjugation, the stability of carbocations may be explained using the concept of inductive effects. The carbon atom is more electronegative than the hydrogen atom with electronegativity values of 2.55 and 2.20, respectively. Therefore, the methyl group is considered electron donating. The t-butyl cation (14) has three electron-donating methyl groups; isopropyl cation (15) has two electron-donating methyl groups; ethyl cation 16 has one electron-donating m...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Series Preface
- Preface
- Editor
- Contributors
- chapter 1: Introduction
- Chapter 2: Nucleophilic Aliphatic Substitution: SN1
- Chapter 3: Nucleophilic Aliphatic Substitution: SN2
- Chapter 4: Electrophilic Addition to Alkenes
- Chapter 5: Electrophilic Aromatic Substitution
- Chapter 6: Rearrangement and Fragmentation Reactions
- Index