
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This book is devoted to the red blood cell membrane, its structure and function, and abnormalities in disease states. It presents a well-documented and well-illustrated comprehensive picture of clinical manifestations of red blood cell disorders.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
MedicinaSubtopic
Biochimica1
Recently Identified Erythrocyte Membrane-Skeletal Proteins and Interactions
Implications for Structure and Function
* National Cancer Institute, National Institutes of Health, Bethesda, Maryland
I. INTRODUCTION
A common approach in modern cell biology and medicine has been to elucidate the various processes and attributes of the living organism through an analysis of the behavior of its most basic constituents. Consequently, much of what is now known about the macromolecular assemblies responsible for cellular function and integrity has arisen from exhaustive in vitro characterization of the individual components that contribute to their structure. This approach, although limited to the test tube, has provided much of the guidance for the construction of conceptual frameworks from which the rules governing cell function and organization can be inferred. Such strategies have found great utility in the elucidation of the factors responsible for the strength and mechanical stability of the human erythrocyte membrane, properties it derives from an anastomosing network of proteins attached to its cytoplasmic surface. Referred to as the “membrane skeleton,” this macromolecular assembly has been the focus of two decades of intense investigation. The cumulative result of these efforts has been the construction of a conceptual model of the membrane skeleton consistent with both the biomedical properties of its individual protein components and the physiological characteristics of its macromolecular form. The operational impact of this construct has been as a schematic paradigm for detailed discussion of membrane skeletal structure in normal and abnormal erythrocytes and for initial studies of the more complex membranes in nonerythroid cells.
The purpose of this chapter will be to review what is known about the more recently identified proteins and interactions of the red blood cell membrane and membrane skeleton. Topics to be covered include red blood cell tropomyosin, myosin, protein 4.9 adducin, and protein 4.2. Finally, the discussion will be extended to include an update on the advances in the current thinking on how known elements of the membrane skeleton, such as ankyrin and protein 4.1, interact with the membrane and how such interactions may be modulated.
II. THE CURRENT CONCEPTUAL MODEL OF THE ERYTHROCYTE MEMBRANE SKELETON
A basic understanding of the anatomy of the red blood cell membrane skeleton is a prerequisite for a clear perception of how these newer elements and interactions may contribute to its structure and function. This chapter will therefore begin with a description of the fundamental aspects of the membrane skeleton (those aspects for which there is general agreement). For a more detailed discussion, the reader should refer to other chapters in this book as well as recent reviews [1–4]. A schematic drawing depicting the molecular organization of the membrane skeleton as well as some of the recently proposed interactions is shown in Figure 1.
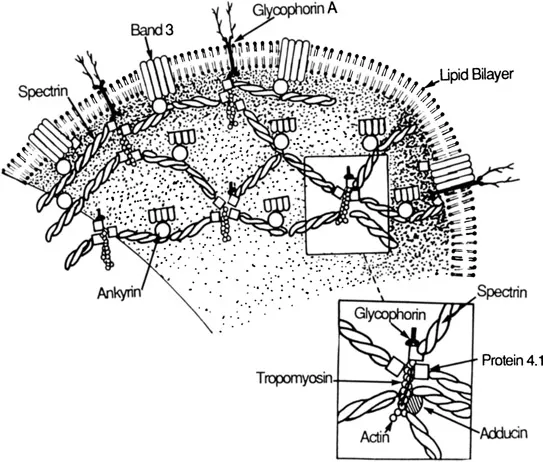
Figure 1 Schematic model for organization of proteins in the human erythrocyte membrane.
The major component of the membrane skeleton is a rather large protein called spectrin [5]. Spectrin is a flexible rodlike molecule present in erythrocytes at ~200,000 copies per cell. It is composed of two similar but nonidentical subunits of 260,000 daltons (spectrin α subunit) and 225,000 daltons (spectrin β subunit) that are intertwined side-to-side to form a heterodimer ~ 100 nm in depth [6]. Spectrin heterodimers are polar molecules with binding sites for specific proteins along different aspects of its structure. A self-association between the head regions of two spectrin heterodimers creates a 200-nm-long tetramer [6,7]. The tetrameric species of spectrin is the predominant form of spectrin in the erythrocyte [8]. Hexamers and other oligomeric forms of spectrin have been observed in vitro and also may contribute to the structure of the membrane skeleton [9,10]. The tail portions of each spectrin heterodimer contain a binding site for actin filaments; spectrin tetramers and oligomers are thus multivalent for actin and can readily cross-link actin filaments [11–14].
Actin in the red blood cell is in the form of short oligomers composed of 12–20 monomers [15,16]. This property of actin is unique to the erythrocyte because in other cells actin is present as more extensively polymerized thin filaments containing hundreds of actin monomers. Calculations that compare the total mass of polymerized actin with the amount of filament ends in the red blood cell imply that there are ~25,000–30,000 actin oligomers per red blood cell [15]. The ratio of spectrin to actin oligomers estimated from this value indicates that each actin oligomer should associate with an average of about six spectrins. Such an arrangement predicts a basic morphology within the membrane skeleton where each actin oligomer is linked by spectrin tetramers to neighboring actin units, thus creating a continuous spectrin-actin lattice beneath the membrane (Figure 1). These predictions have been confirmed by high-resolution electron micrographs of isolated erythrocyte membrane skeletons [17–19]. The micrographs show a highly repeated and remarkably regular organization of the spectrin-actin complexes in which each complex is interlinked to adjacent complexes by multiple spectrin tetramers. In remarkable agreement with predictions based on relative amounts of spectrin and actin, five to eight spectrin tetramers are gathered about each actin core (Figure 1).
The major high-affinity attachment site between the membrane skeleton and the overlying lipid bilayer is provided by ankyrin [20–23,103]. Erythrocyte ankyrin is a slightly asymmetric globular protein with a molecular weight of 215,000 and it is present in the erythrocyte at 100,000 copies per cell [22]. It contains separate binding sites for both spectrin and the anion transporter (band 3) that is the major integral membrane protein of the red blood cell [24]. The membrane skeleton is thus tethered to the lipid bilayer by a stable spectrinankyrin-anion transporter linkage. A binding site for ankyrin is located on the beta subunit of spectrin ~20 nm from its head region [25]. The ratio of spectrin dimers to ankyrin in the red blood cell suggests that, on average, each spectrin tetramer is linked to the anion transporter by a single ankyrin.
Spectrin-actin interactions are very weak independently, but are greatly bilized by the presence of protein 4.1 [13,14]. Protein 4.1 and other proteins such as 4.9 and 4.2 were named based on relative mobility on SDS-polyacryl-amide electrophoresis gels [26]. Protein 4.1 has a molecular weight of approximately 78,000 daltons, and is present in red cells at about 200,000 copies per cell. The actin-binding tail regions of spectrin contain binding sites for protein 4.1 on each subunit [25–27,28]. Protein 4.1 increase the binding of spectrin to actin by an allosteric mechanism that increases spectrin affinity for actin. Alternately the participation of protein 4.1 in the formation of more stable ternary complexes with spectrin and actin would also be expected to enhance spectrinactin interactions [29,30]. In any case, the actual molecular mechanism of protein 4.1 action remains obscure. The 1:1 ratio between the amount of spectrin dimers in the red blood cell and that of protein 4.1 implies that every spectrin in the red blood cell could have a protein 4.1 bound. However, because in vitro experiments suggest that each spectrin is capable of interacting with two molecules of protein 4.1 and that specific interactions exist between protein 4.1 and other proteins (see below), this impression is likely to be an oversimplification.
III. RECENTLY IDENTIFIED ERYTHROCYTE MEMBRANE-SKELETAL PROTEINS
A. Erythrocyte Tropomyosin
Tropomyosin is a well-conserved rodlike molecule present in many types of eukaryotic cells. Its functions are best understood in the contractile apparatus of muscle where, as a thin filament component, tropomyosin binds along both grooves of the actin filament helix to confer calcium sensitivity on the interaction between actin and myosin in the presence of troponin [31]. Tropomyosin is composed of two α helical subunits that are wrapped around each other in an elongated coiled-coil structure. In nonmuscle cells these subunits are slightly smaller than their counterpart in muscle, with molecular weights of 30,000 as compared with 35,000. Erythrocyte tropomyosin was first identified as polypeptides cross-reacting with chicken gizzard smooth-muscle tropomyosin that were present in red blood cell membranes isolated in the presence of millimolar concentrations of magnesium [32]. The erythroid form of tropomyosin is present as a 3:1 mixture of 29,000 dalton polypeptides and 27,000 polypeptides, respectively. These polypeptides have properties shared by other forms of tropomyosin, including: (a) Stokes radius, sedimentation coefficient, and high calculated frictional ratio; (b) stability to low pH and high temperature; (c) amino acid composition; (d) anomalous migration on sodium dodecyl sulfate (SDS) polyacrylamide gels in the presence of urea [32].
Erythrocyte tropomyosin is distinct from the other nonmuscle forms in that it binds well to actin at physiological concentrations of magnesium (2–3 mM), conditions where the nonmuscle forms bind weakly or not at all. In addition, the erythroid form of tropomyosin binds actin with a cooperativity that is not seen with its nonmuscle counterparts. This property suggests that red blood cell tropomyosin dimers may have the ability to bind each other in a tail-to-tail fashion, as has been proposed for the muscle forms of tropomyosin, which not only show cooperative binding to actin, but, like the erythroid form, bind well to actin filaments at lower concentrations of magnesium.
In vitro binding assays with erythrocyte tropomyosin indicate that a heterodimer of 27,000 and 29,000-dalton polypeptides may have both a higher affinity for actin and a lower magnesium requirement than the 29,000-dalton form [32]. It will be of interest in future work to separate the 27,000 and 29,000-dalton polypeptides and evaluate activities of homo- and heterodimers of these polypeptides.
Tropomyosin from other sources make actin filaments more rigid and resistant to depolymerization [33–36]. One function of erythrocyte tropomyosin may be to stabilize membrane-bound forms of actin. This property could have great importance in the biogenesis of the short actin filaments during erythroid differentiation. Because the intracellular concentrations of magnesium increase when the erythrocyte is deoxygenated [37,38], tropomyosin may provide a mechanism through which the stability of the membrane skeleton can be influenced by the oxygenation state of the red blood cell. Calmodulin, a protein homologous to troponin C, is present at micromolar concentrations in the erythrocyte [39,40] and evidence exists for an erythroid form of troponin I [41]. Considering these observations, the presence of tropomyosin in the red blood cell implies the possible existence of a troponin T-like molecule that may act with tropomyosin and the other analogues of the troponin complex to provide a calcium-sensitive means of regulating the interaction of actin with other actin-binding proteins within the membrane skeleton. This possibility is supported by the observation that tropomyosin is able to inhibit the association of red blood spectrin with actin [42].
B. Erythrocyte Myosin
Erythrocyte myosin was identified in erythrocytes as a polypeptide on SDS-polyacrylamide gels that cross-reacted with antibodies against platelet myosin [43]. Its purification from red blood cells confirms and extends earlier reports of myosinlike proteins and adenosine triphosphatase (ATPase) activity in erythrocyte-membrane-derived extracts [44,45]. The protein is remarkably similar to the nonmuscle myosins found in platelets and other tissues. Rotary shadowed micrographs of red blood cell myosin reveal a familiar 150-nm-long molecule with two globular heads and a rodlike tail. Other properties the erythrocyte myosin has in common with its nonmuscle counterparts include: (a) a calcium-activated and magnesium-inhibited ATPase activity; (b) high ATPase activity in the presence of...
Table of contents
- Cover
- Half Title
- Series Page
- Title Page
- Copyright Page
- Dedication Page
- Series Introduction
- Preface
- Contributors
- Contents
- 1. Recently Identified Erythrocyte Membrane-Skeletal Proteins and Interactions: Implications for Structure and Function
- 2. Structure and Function of the Glucose Transporter
- 3. Intermediate Filament Expression in Erythroid Differentiation and Morphogenesis
- 4. The Erythrocyte Cytoskeleton in Hereditary Elliptocytosis and Spherocytosis
- 5. Spectrin Genes
- 6. Na,K-ATPase Structure
- 7. Characterization of the Gene Coding for Human Erythrocyte Protein 4.1: Implications for Understanding Hereditary Elliptocytosis
- 8. Regulation of Protein 4.1-Membrane Associations by a Phosphoinositide
- 9. Interaction of Native and Denatured Hemoglobins with Band 3: Consequences for Erythrocyte Structure and Function
- 10. Ultrastructure and Function of Membrane Skeleton
- 11. The Biochemistry of the Antigens of the Red Blood Cell Membrane
- 12. The Transferrin Receptor
- 13. Red Blood Cell Membrane Protein and Lipid Diffusion
- 14. Red Blood Cell Shape
- 15. Viscoelastic Properties and Rheology
- 16. Active Transport of Sodium and Potassium
- 17. The Plasma Membrane Calcium Pump: The Red Blood Cell as a Model
- 18. Passive Cation Transport
- 19. Anion Transport
- 20. The Kinetics and Thermodynamics of Glucose Transport in Human Erythrocytes
- 21. Nucleoside Transport
- 22. Regulated Transport: The Response of Ion Transport Pathways to Physiological Stimuli
- 23. Ion Transport in Red Blood Cell Disorders
- 24. Partial Deficiencies of Erythrocyte Spectrin in Hereditary Spherocytosis
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Red Blood Cell Membranes by Peter Agre in PDF and/or ePUB format, as well as other popular books in Medicina & Biochimica. We have over one million books available in our catalogue for you to explore.