
eBook - ePub
Frontiers in Clinical Drug Research - Hematology: Volume 3
Atta-ur-Rahman
This is a test
Share book
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Frontiers in Clinical Drug Research - Hematology: Volume 3
Atta-ur-Rahman
Book details
Book preview
Table of contents
Citations
About This Book
Frontiers in Clinical Drug Research – Hematology is a book series that brings updated reviews to readers interested in learning about advances in the development of pharmaceutical agents for the treatment of hematological disorders. The scope of the book
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Frontiers in Clinical Drug Research - Hematology: Volume 3 an online PDF/ePUB?
Yes, you can access Frontiers in Clinical Drug Research - Hematology: Volume 3 by Atta-ur-Rahman in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Clinical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Topic
Physical SciencesSubtopic
Clinical ChemistryRole of Immunomodulatory Drugs in the Treatment of Lymphoid and Myeloid Malignancies
Ota Fuchs*
Institute of Hematology and Blood Transfusion, U Nemocnice 1, 128 20 Prague 2, Czech Republic
Abstract
Immunomodulatory drugs (IMiDs) or cereblon (CRBN) binding drugs such as thalidomide, lenalidomide and pomalidomide have similar structures and mechanism of action. Lenalidomide (CC5013, Revlimid®) was approved by the US FDA and the EMA for the treatment of multiple myeloma (MM) patients, low or intermediate-1 risk transfusion-dependent myelodysplastic syndrome (MDS) with chromosome 5q deletion [del(5q)] and relapsed and/or refractory mantle cell lymphoma following bortezomib. Lenalidomide has also been studied in clinical trials and has shown promising activity in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL). Lenalidomide has anti-inflammatory effects and inhibits angiogenesis. Pomalidomide (CC4047, Imnovid® [EU], Pomalyst® [USA]) has been recently approved by the US FDA and the EMA for patients with relapsed or refractory MM who have received at least two prior therapies, including lenalidomide and bortezomib. Cereblon seems to have an important role in IMiDs action in both lymphoid and myeloid hematological malignancies and has been identified as a direct molecular target for anti-neoplastic activities of IMiDs. Lenalidomide binds to cereblon (CRBN) which acts as the substrate receptor of a cullin-4 really interesting new gene (RING) E3 ubiquitin ligase CRL4CRBN. This E3 ubiquitin ligase in the absence of lenalidomide ubiquitinates CRBN itself and the other component of CRL4CRBN complex, DNA damage binding protein 1 (DDB1) but in the presence of lenalidomide it changes its specificity and ubiquitinates two transcription factors, IKZF1 (Ikaros) and IKZF3 (Aiolos), and casein kinase 1α (CK1α) and degrades them in proteasomes. Both these transcription factors IKZF1 and IKZF3 are important for the viability of MM cells. IKZF1 induces transcription from interferon regulatory factor 4 gene (IRF4) promoter and from MYC gene promoter in B cells. IKZF1/3 repress the interleukin 2 gene (IL-2) promoter in T cells. In such a way, a decline in IKZF1/3 levels explains how IMiDs stimulate the immune system and degrade B cell function. Low CRBN expression correspond with drug resistance in MM cells. CK1α is a serine/threonine kinase and the CK1α gene (CSNK1A1) is located on 5q32 in commonly deleted region (CDR) in del(5q) MDS. Inhibition of CK1α sensitizes del(5q) MDS cells to lenalidomide. CK1α mediates also the survival of malignant plasma cells in MM. Though, the inhibition of CK1α is a potential novel therapy not only in del(5q) MDS but also in MM. High level of full length CRBN mRNA in mononuclear cells of bone marrow and of peripheral blood seems to be necessary for successful lenalidomide treatment of del(5q) MDS. Bone marrow aspirates of MDS patients who responded to lenalidomide showed before treatment decreased expression of the set of genes needed for erythroid differentiation. Lenalidomide seemed to overcome differentiation block in non-del(5q) low risk MDS patients with decreased expression of these genes compared to the non-responders but this suggestion was not confirmed.
Keywords: Cereblon, Casein kinase 1α1, Cullin 4-containing RING E3 ubiquitin ligase complex, Ikaros family, Immunomodulatory drugs, Lenalidomide, Pomalidomide, Multiple myeloma, Del(5q) MDS, Mantle lymphoma, Proteasome.
* Corresponding author Ota Fuchs: Institute of Hematology and Blood Transfusion, U Nemocnice 1, 12820 Prague 2, Czech Republic; Tel: 420221977512; Fax: 420221977370; E-mail: [email protected]
INTRODUCTION
IMiDs or cereblon binding drugs, thalidomide and its derivatives, lenalidomide and pomalidomide, are important immunotherapeutic drugs. Chemical structure of these drugs is shown in Fig. (1). Thalidomide, 2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (Thalomid), the first IMiD, was originally synthesized in Germany from α-phtaloylisoglutamine, to be used as sedative and antiemetic drug [1]. In 1957, after a short period of preclinical studies, thalidomide was approved for the treatment of nausea in pregnant women. The appearance of malformations such as phocomelia in the newborn banned its use three years later [2-6]. Despite its history as a human teratogen, thalidomide is now used for a treatment of cancer and inflammatory diseases. The US Food and Drug Administration (FDA) approved thalidomide in 1998 for the treatment of erythema nodosum leprosum.
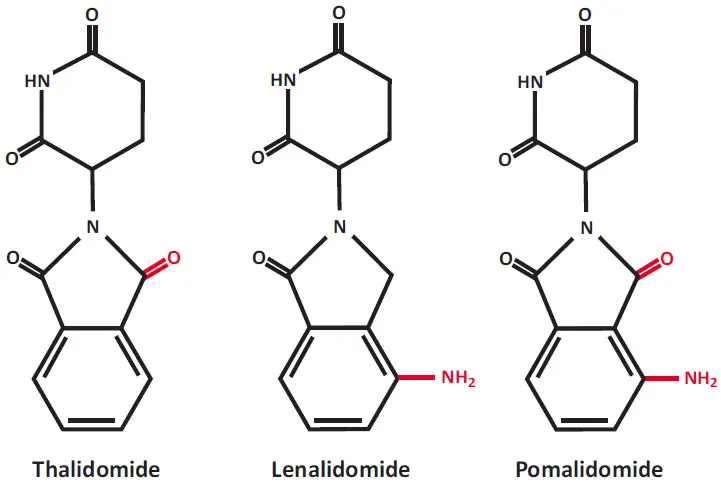
Chemical structures of immunomodulatory drugs (IMiDs), now also known as a cereblon-binding drugs. Lenalidomide and pomalidomide are synthetic compounds derived by modifying the chemical structure of thalidomide.
In 2006, FDA granted approval for thalidomide in combination with dexame-thasone for the treatment of multiple myeloma (MM) patients [7-9]. Drug combinations containing thalidomide are still used for the MM patients therapy [10-12]. Thalidomide therapy is efficient in non-Hodgkin lymphomas (NHL) [13, 14], mainly in relapsed mantle cell lymphoma [15, 16], and in chronic lymphocytic leukemia (CLL) [17-19]. A small part of transfusion-dependent MDS patients achieved transfusion independence by treatment with thalidomide [20-28].
Lenalidomide and pomalidomide are analogs of thalidomide with strong immunomodulatory, anti-angiogenic and direct neoplastic cell inhibitory activities [29-64]. Lenalidomide was developed in order to avoid thalidomide side effects (sedation, constipation and peripheral neuropathy), and to increase anti-tumor efficacy [30]. Lenalidomide (CC-5013, Revlimid) shares some structural features and biological properties with thalidomide but is safer and more potent than thalidomide. Lenalidomide is not teratogenic in rabbit models [30]. Lenalidomide also is a more potent stimulator of T-cell proliferation and cytokine production (γ-interferon and interleukin-2) [35]. Pomalidomide (CC-4047, Pomalyst, Imnovid) is indicated for patients with MM who have received at least two prior therapies including lenalidomide and a proteasome inhibitor bortezomib and have demonstrated disease progression [65-76]. Pomalidomide and lenalidomide have mild adverse effects. Pomalidomide is more potent than both thalidomide and lenalidomide with regard to T-cell co-stimulation [31].
CRBN (see Fig. 2) is a substrate receptor of the E3 ubiquitin ligase complex (CRL4CRBN) and the target of thalidomide teratogenicity [64, 77-83]. The CRL4CRBN protein ligase complex functions in the ubiquitination and subsequent degradation of target proteins in proteasomes and is required for cellular protein homeostasis. The ubiquitin-proteasome system (UPS) is the non-lysosomal protein degradation pathway that acts in cell cycle regulation, cell differentiation, response to stress, transcription regulation, DNA repair and programmed cell death [84-101]. The pathogenesis of diseases, such as cancer, neurological, autoimmune, genetic and metabolic disorders is connected with the deregulation of the UPS [88, 91, 97-99]. Thalidomide and its chemical relatives binding to CRBN changes the target specificity of the CRL4CRBN protein ligase complex (Fig. 3). The CRL4CRBN targets proteins...