
eBook - ePub
Frontiers in Clinical Drug Research - Hematology: Volume 3
Atta-ur-Rahman
This is a test
Buch teilen
- English
- ePUB (handyfreundlich)
- Über iOS und Android verfügbar
eBook - ePub
Frontiers in Clinical Drug Research - Hematology: Volume 3
Atta-ur-Rahman
Angaben zum Buch
Buchvorschau
Inhaltsverzeichnis
Quellenangaben
Über dieses Buch
Frontiers in Clinical Drug Research – Hematology is a book series that brings updated reviews to readers interested in learning about advances in the development of pharmaceutical agents for the treatment of hematological disorders. The scope of the book
Häufig gestellte Fragen
Wie kann ich mein Abo kündigen?
Gehe einfach zum Kontobereich in den Einstellungen und klicke auf „Abo kündigen“ – ganz einfach. Nachdem du gekündigt hast, bleibt deine Mitgliedschaft für den verbleibenden Abozeitraum, den du bereits bezahlt hast, aktiv. Mehr Informationen hier.
(Wie) Kann ich Bücher herunterladen?
Derzeit stehen all unsere auf Mobilgeräte reagierenden ePub-Bücher zum Download über die App zur Verfügung. Die meisten unserer PDFs stehen ebenfalls zum Download bereit; wir arbeiten daran, auch die übrigen PDFs zum Download anzubieten, bei denen dies aktuell noch nicht möglich ist. Weitere Informationen hier.
Welcher Unterschied besteht bei den Preisen zwischen den Aboplänen?
Mit beiden Aboplänen erhältst du vollen Zugang zur Bibliothek und allen Funktionen von Perlego. Die einzigen Unterschiede bestehen im Preis und dem Abozeitraum: Mit dem Jahresabo sparst du auf 12 Monate gerechnet im Vergleich zum Monatsabo rund 30 %.
Was ist Perlego?
Wir sind ein Online-Abodienst für Lehrbücher, bei dem du für weniger als den Preis eines einzelnen Buches pro Monat Zugang zu einer ganzen Online-Bibliothek erhältst. Mit über 1 Million Büchern zu über 1.000 verschiedenen Themen haben wir bestimmt alles, was du brauchst! Weitere Informationen hier.
Unterstützt Perlego Text-zu-Sprache?
Achte auf das Symbol zum Vorlesen in deinem nächsten Buch, um zu sehen, ob du es dir auch anhören kannst. Bei diesem Tool wird dir Text laut vorgelesen, wobei der Text beim Vorlesen auch grafisch hervorgehoben wird. Du kannst das Vorlesen jederzeit anhalten, beschleunigen und verlangsamen. Weitere Informationen hier.
Ist Frontiers in Clinical Drug Research - Hematology: Volume 3 als Online-PDF/ePub verfügbar?
Ja, du hast Zugang zu Frontiers in Clinical Drug Research - Hematology: Volume 3 von Atta-ur-Rahman im PDF- und/oder ePub-Format sowie zu anderen beliebten Büchern aus Ciencias físicas & Química clínica. Aus unserem Katalog stehen dir über 1 Million Bücher zur Verfügung.
Information
Thema
Ciencias físicasThema
Química clínicaRole of Immunomodulatory Drugs in the Treatment of Lymphoid and Myeloid Malignancies
Ota Fuchs*
Institute of Hematology and Blood Transfusion, U Nemocnice 1, 128 20 Prague 2, Czech Republic
Abstract
Immunomodulatory drugs (IMiDs) or cereblon (CRBN) binding drugs such as thalidomide, lenalidomide and pomalidomide have similar structures and mechanism of action. Lenalidomide (CC5013, Revlimid®) was approved by the US FDA and the EMA for the treatment of multiple myeloma (MM) patients, low or intermediate-1 risk transfusion-dependent myelodysplastic syndrome (MDS) with chromosome 5q deletion [del(5q)] and relapsed and/or refractory mantle cell lymphoma following bortezomib. Lenalidomide has also been studied in clinical trials and has shown promising activity in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL). Lenalidomide has anti-inflammatory effects and inhibits angiogenesis. Pomalidomide (CC4047, Imnovid® [EU], Pomalyst® [USA]) has been recently approved by the US FDA and the EMA for patients with relapsed or refractory MM who have received at least two prior therapies, including lenalidomide and bortezomib. Cereblon seems to have an important role in IMiDs action in both lymphoid and myeloid hematological malignancies and has been identified as a direct molecular target for anti-neoplastic activities of IMiDs. Lenalidomide binds to cereblon (CRBN) which acts as the substrate receptor of a cullin-4 really interesting new gene (RING) E3 ubiquitin ligase CRL4CRBN. This E3 ubiquitin ligase in the absence of lenalidomide ubiquitinates CRBN itself and the other component of CRL4CRBN complex, DNA damage binding protein 1 (DDB1) but in the presence of lenalidomide it changes its specificity and ubiquitinates two transcription factors, IKZF1 (Ikaros) and IKZF3 (Aiolos), and casein kinase 1α (CK1α) and degrades them in proteasomes. Both these transcription factors IKZF1 and IKZF3 are important for the viability of MM cells. IKZF1 induces transcription from interferon regulatory factor 4 gene (IRF4) promoter and from MYC gene promoter in B cells. IKZF1/3 repress the interleukin 2 gene (IL-2) promoter in T cells. In such a way, a decline in IKZF1/3 levels explains how IMiDs stimulate the immune system and degrade B cell function. Low CRBN expression correspond with drug resistance in MM cells. CK1α is a serine/threonine kinase and the CK1α gene (CSNK1A1) is located on 5q32 in commonly deleted region (CDR) in del(5q) MDS. Inhibition of CK1α sensitizes del(5q) MDS cells to lenalidomide. CK1α mediates also the survival of malignant plasma cells in MM. Though, the inhibition of CK1α is a potential novel therapy not only in del(5q) MDS but also in MM. High level of full length CRBN mRNA in mononuclear cells of bone marrow and of peripheral blood seems to be necessary for successful lenalidomide treatment of del(5q) MDS. Bone marrow aspirates of MDS patients who responded to lenalidomide showed before treatment decreased expression of the set of genes needed for erythroid differentiation. Lenalidomide seemed to overcome differentiation block in non-del(5q) low risk MDS patients with decreased expression of these genes compared to the non-responders but this suggestion was not confirmed.
Keywords: Cereblon, Casein kinase 1α1, Cullin 4-containing RING E3 ubiquitin ligase complex, Ikaros family, Immunomodulatory drugs, Lenalidomide, Pomalidomide, Multiple myeloma, Del(5q) MDS, Mantle lymphoma, Proteasome.
* Corresponding author Ota Fuchs: Institute of Hematology and Blood Transfusion, U Nemocnice 1, 12820 Prague 2, Czech Republic; Tel: 420221977512; Fax: 420221977370; E-mail: [email protected]
INTRODUCTION
IMiDs or cereblon binding drugs, thalidomide and its derivatives, lenalidomide and pomalidomide, are important immunotherapeutic drugs. Chemical structure of these drugs is shown in Fig. (1). Thalidomide, 2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione (Thalomid), the first IMiD, was originally synthesized in Germany from α-phtaloylisoglutamine, to be used as sedative and antiemetic drug [1]. In 1957, after a short period of preclinical studies, thalidomide was approved for the treatment of nausea in pregnant women. The appearance of malformations such as phocomelia in the newborn banned its use three years later [2-6]. Despite its history as a human teratogen, thalidomide is now used for a treatment of cancer and inflammatory diseases. The US Food and Drug Administration (FDA) approved thalidomide in 1998 for the treatment of erythema nodosum leprosum.
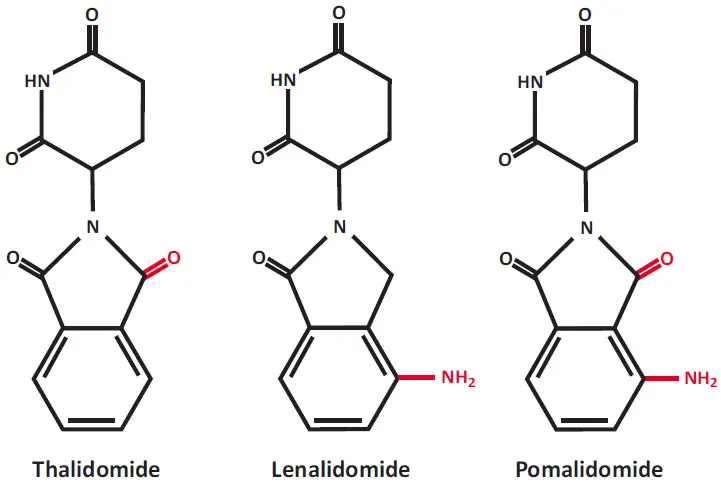
Chemical structures of immunomodulatory drugs (IMiDs), now also known as a cereblon-binding drugs. Lenalidomide and pomalidomide are synthetic compounds derived by modifying the chemical structure of thalidomide.
In 2006, FDA granted approval for thalidomide in combination with dexame-thasone for the treatment of multiple myeloma (MM) patients [7-9]. Drug combinations containing thalidomide are still used for the MM patients therapy [10-12]. Thalidomide therapy is efficient in non-Hodgkin lymphomas (NHL) [13, 14], mainly in relapsed mantle cell lymphoma [15, 16], and in chronic lymphocytic leukemia (CLL) [17-19]. A small part of transfusion-dependent MDS patients achieved transfusion independence by treatment with thalidomide [20-28].
Lenalidomide and pomalidomide are analogs of thalidomide with strong immunomodulatory, anti-angiogenic and direct neoplastic cell inhibitory activities [29-64]. Lenalidomide was developed in order to avoid thalidomide side effects (sedation, constipation and peripheral neuropathy), and to increase anti-tumor efficacy [30]. Lenalidomide (CC-5013, Revlimid) shares some structural features and biological properties with thalidomide but is safer and more potent than thalidomide. Lenalidomide is not teratogenic in rabbit models [30]. Lenalidomide also is a more potent stimulator of T-cell proliferation and cytokine production (γ-interferon and interleukin-2) [35]. Pomalidomide (CC-4047, Pomalyst, Imnovid) is indicated for patients with MM who have received at least two prior therapies including lenalidomide and a proteasome inhibitor bortezomib and have demonstrated disease progression [65-76]. Pomalidomide and lenalidomide have mild adverse effects. Pomalidomide is more potent than both thalidomide and lenalidomide with regard to T-cell co-stimulation [31].
CRBN (see Fig. 2) is a substrate receptor of the E3 ubiquitin ligase complex (CRL4CRBN) and the target of thalidomide teratogenicity [64, 77-83]. The CRL4CRBN protein ligase complex functions in the ubiquitination and subsequent degradation of target proteins in proteasomes and is required for cellular protein homeostasis. The ubiquitin-proteasome system (UPS) is the non-lysosomal protein degradation pathway that acts in cell cycle regulation, cell differentiation, response to stress, transcription regulation, DNA repair and programmed cell death [84-101]. The pathogenesis of diseases, such as cancer, neurological, autoimmune, genetic and metabolic disorders is connected with the deregulation of the UPS [88, 91, 97-99]. Thalidomide and its chemical relatives binding to CRBN changes the target specificity of the CRL4CRBN protein ligase complex (Fig. 3). The CRL4CRBN targets proteins...