
eBook - ePub
Fundamentals of Medicinal Chemistry and Drug Metabolism
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Fundamentals of Medicinal Chemistry and Drug Metabolism
About this book
The primary objective of this 4-volume book series is to educate PharmD students on the subject of medicinal chemistry. The book set serves as a reference guide to pharmacists on aspects of chemical basis of drug action. This first volume of the series is
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Fundamentals of Medicinal Chemistry and Drug Metabolism by M. O. Faruk Khan,v Philip, M. O. Faruk Khan, v Philip in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.
Information
Drug Metabolism
Rahmat Talukder1, Ashok Philip2, M. O. Faruk Khan3, *
1 Department of Pharmaceutical Sciences, University of Texas at Tyler College of Pharmacy, Tyler, TX, USA
2 Department of Pharmaceutical Sciences, Union University School of Pharmacy, Jackson, TN, USA
3 Department of Pharmaceutical Sciences and Research, Marshall University School of Pharmacy, Huntington, WV, USA
Abstract
This chapter is a detailed account of drug metabolism, prodrugs and related terminology that are critical knowledge base for pharmacist and pharmacy education. After study of this chapter, students will be able to:
• Comprehend the fundamental concepts of drug metabolism
• Describe the significance of drug metabolism
• Identify key enzymes involved and the sites of drug metabolism
• Explain phase I and phase II metabolic pathways, including:
♦ Phase I (Functionalization)
- Oxidation of aromatic moieties, olefins, benzylic & allylic C atoms and α-C of C=O and C=N, aliphatic and alicyclic C, C-heteroatom system, C-N (N-dealkylation, N-oxide formation, N-hydroxylation), C-O (O-dealkylation), C-S (S-dealkylation, S-oxidation, desulfuration), alcohols and aldehydes, and miscellaneous oxidative reactions
- Reduction of aldehydes and ketones, Nitro and azo compounds, and miscellaneous reductive metabolisms
- Hydrolytic reactions of esters and amides, epoxides and arene oxides by epoxide hydrase
♦ Phase II (Conjugation)
- Glucuronic acid conjugation, sulfate conjugation, glycine and other amino acid, glutathione or mercapturic acid, acetylation, methylation
- Define and differentiate between prodrug, soft drug and antedrugs
- Discuss metabolic routes of some individual drugs
• Describe the significance of drug metabolism
• Identify key enzymes involved and the sites of drug metabolism
• Explain phase I and phase II metabolic pathways, including:
♦ Phase I (Functionalization)
- Oxidation of aromatic moieties, olefins, benzylic & allylic C atoms and α-C of C=O and C=N, aliphatic and alicyclic C, C-heteroatom system, C-N (N-dealkylation, N-oxide formation, N-hydroxylation), C-O (O-dealkylation), C-S (S-dealkylation, S-oxidation, desulfuration), alcohols and aldehydes, and miscellaneous oxidative reactions
- Reduction of aldehydes and ketones, Nitro and azo compounds, and miscellaneous reductive metabolisms
- Hydrolytic reactions of esters and amides, epoxides and arene oxides by epoxide hydrase
♦ Phase II (Conjugation)
- Glucuronic acid conjugation, sulfate conjugation, glycine and other amino acid, glutathione or mercapturic acid, acetylation, methylation
- Define and differentiate between prodrug, soft drug and antedrugs
- Discuss metabolic routes of some individual drugs
Keywords: Antedrug, Conjugation, Drug interactions, Drug metabolism, Enterohepatic circulation, First pass effect, Phase I metabolism, Phase II metabolism, Prodrug, Soft drug.
* Corresponding author M.O. Faruk Khan: Department of Pharmaceutical Sciences and Research, Marshall University School of Pharmacy, Huntington, WV, USA; Tel: 304-696-3094; Fax: 304-696-7309;
E-mail: [email protected]
INTRODUCTORY CONCEPTS
Roles Played by Drug Metabolism
Metabolism is one of four pharmacokinetic parameters, i.e., absorption, distribution, metabolism and excretion (ADME), with metabolism and excretion together considered as elimination. Kidney, the major excretory organ, primarily excretes polar compounds and with respect to drugs, those that are extensively ionized at urinary pH. On the other hand, the kidney is unable to excrete drugs with high lipid water partition coefficient (LWPC), drugs that are unionized at urinary pH. In general, as a result of metabolism, drugs become more polar, ionizable and thus more water soluble with enhanced elimination. It also effects deactivation and thus detoxification. In some instances, the drugs are metabolically activated, referred to as Prodrugs, especially by phase I mechanism. Overall, metabolism may result in one of the following outcomes: 1) drug inactivation or detoxification, 2) metabolite(s) with similar activity, 3) metabolite with different activity, 4) drug intoxication, or 5) drug activation.
1. Drug Inactivation or Detoxification
When metabolism of a drug or a toxic substance results in the formation of an inactive metabolite, it is termed as drug inactivation or detoxification, respectively. For example, the glucuronide conjugation of the p-amino group (N4) or the N atom of the sulfonamide group (N1) of sulfa drugs leads to inactivation. On the other hand, the sulfate conjugation of the toxic phenol leads to detoxification (Fig. 1). In general, CYP [1], UDP-glucuronosyltransferases [2] and glutathione S-transferases [3] play important roles in drug inactivation and detoxifications.
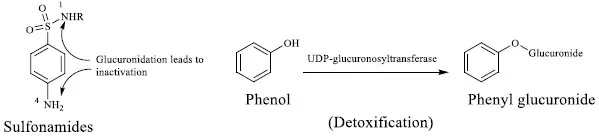
Inactivation and detoxification reactions.
2. Similar Activity
Metabolism may result in a metabolite with similar activity, but with different potency and/or pharmacokinetic and safety profiles. For example, diazepam is an anxiolytic agent with longer duration of action compared to its active metabolites temazepam and oxazepam (Fig. 2) [4]. Acetaminophen is an active metabolite of phenacetin with improved pharmacological and toxicological profiles, and so are fexofenadine and desloratadine [5].
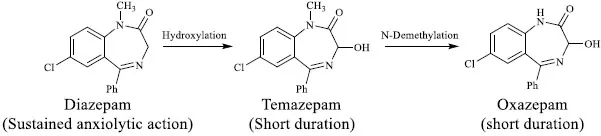
Metabolism of diazepam leading to similarly active metabolites.
3. Different Activity
Drug metabolism may also result in a metabolite with entirely new pharmacologic activity. For example, the hydralazine derivatives of monoamine oxidase inhibitors were developed based on the structural modification of isoniazid. The observation that isoniazid made patients euphoric led to develop its isopropyl (lipophilic) analog to deliver it into the brain better. The isopropyl derivative of isoniazid was named iproniazid (Fig. 3) that was approved in 1958 as an antidepressant agent [6].
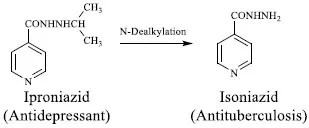
Iproniazid is antidepressant but its metabolite isoniazid is antitubercular agent.
4. Intoxication
Sometimes metabolism may lead to the formation of toxic products, generally as a result of phase I metabolism. For example, acetylhydrazine and isopropylhydra-zine, the metabolites of isoniazid and iproniazid, respectively are hepatotoxic. The CYP450 enzymes are responsible for the production of these toxic metabolites, which are extremely reactive acetylating and alkylating agents (Fig. 4) [7]. Although the phase 2 metabolism (conjugation) is considered to be involved in detoxification reaction, it can also lead to toxic metabolites. For example, some N-glucuronides of arylamines are involved in bladder and colon cancers. Similarly, GSH conjugation, the most important detoxification mechanism in the humans against xenobiotics, may also cause renal toxicity due to the high level of γ-glutamyl transpeptidase activity in kidneys. For example, the GSH conjugate of 2-bromohydroquinone (in the liver) is converted to a nephrotoxic met...
Table of contents
- Welcome
- Table of Contents
- Title
- BENTHAM SCIENCE PUBLISHERS LTD.
- FOREWORD
- Preface
- List of Contributors
- Introduction
- Review of Bioorganic Chemistry
- Acid-base Chemistry and Salt Formation
- Solubility and Lipid-Water Partition Coefficient
- Isosteric and Spatial Considerations of Drugs
- Fundamentals of Drug Action
- Drug Metabolism
- Biosynthetic Pathways Frequently Targeted by Pharmaceutical Intervention