![]()
1 Muscle structure and function
Skeletal muscle tissue is the most abundant tissue in humans and in vertebrates in general. Given its vital role in mechanical activities related to breathing, acquiring food or to escape predators, this is not surprising. In “athlete” animals, such as horses or dogs, skeletal muscle tissue can be as much as 45 per cent of the body mass. The physiology and structure of skeletal muscle tissue as a mechanically active tissue has been well characterized and will be described below. Providing contractile force for movement has historically been seen as the main – if not the only – function of muscle. More recently, it has become increasingly clear that skeletal muscle tissue has many more tasks. Skeletal muscle tissue plays a key role in regulating energy balance of the whole organism (Stefanyk and Dyck, 2010). Further, working muscle tissue secretes myokines and thus effectively functions as an endocrine organ. As such, muscle tissue is involved in tissue crosstalk, mainly between muscle, adipose tissue and brain. Activated muscle is held responsible for antiinflammatory effects important in the control of diabetes type 2, cardiovascular disease, cancer and neurodegenerative diseases (Pedersen, 2011).
As described in this chapter, the bulk of skeletal muscle tissue consists of contractile proteins. We usually see these proteins as responsible for muscle shortening (i.e. concentric contraction). It is often overlooked that in natural locomotion, just as often, muscles do not shorten during activation, but rather resist lengthening (i.e. perform eccentric contractions). Eccentric muscle activity serves two major purposes. Muscles work eccentrically to dissipate energy for decelerating the body (e.g. when walking downhill). Eccentric contractions are further important during the early stance phase of locomotion. In this case, eccentrically contracting muscles allow for converting kinetic and potential energy into elastic strain energy of tendons and aponeuroses. This energy is then regained during limb support and minimizes muscle work, and thus energy requirements, in locomotion (Biewener, 2006). In the chapters of this book, we will systematically look into the specifics of the physiology and the application of eccentric contractions both in rehabilitation and in sports. The first chapter contains an overview of the structural underpinnings of muscle contraction phenomena with a focus on recent concepts of relevance to eccentric contractions. This chapter is thus a very dense summary of currently available knowledge useful for the comprehension of eccentric muscle phenomena in the context of modern muscle physiology. The reader is assumed to have an elementary background knowledge of muscle structure and function.
1.1 The contractile machinery of muscle fibres
Overview: Muscle tissue is often seen as consisting mainly of actin and myosin together with their regulatory proteins in sarcomeric organization and with the purpose of generating contractile force. This view has been modified by the broader view that a vast number of additional proteins serve specific, unique and necessary functions in muscle development, maintenance and plasticity. Prominent among these proteins is titin, seen as the “third” filament, mainly responsible for the passive, elastic properties of muscle tissue. Together with nebulin and obscurin, titin is seen as a scaffold protein helping to maintain sarcomeres, its constituent and associated proteins, as well as the t- and l-tubular system in register. Many muscle structural proteins contain domains associated with signalling molecules that endow muscle with phenotypicplasticity (i.e. the ability to adapt to specific mechanical stress and to repair after injury).
The basic building block of muscle tissue is the muscle cell also called muscle fibre. Muscle fibres are the mechanically active component of muscle tissue and generally make up some 80–90 per cent of the volume of a skeletal muscle. The rest of the volume is taken up by interstitial tissue, the main component of which is connective tissue (i.e. fibroblasts and the collagen fibrils they produce). The main task of muscle connective tissue is to bind muscle fibres together and to transfer tension produced by muscle fibres to tendons and thus to the skeleton. Embedded in the connective tissue are nerves and the vasculature supplying muscle fibres.
Muscle fibres are enormously large as compared to most other cells of the body. They can be some 30–80 μm in diameter and up to 10 cm long, when most other cells of our body have diameters around 7–10 μm. While almost all cells of our body contain only one nucleus, muscle fibres contain thousands of nuclei, which are, in healthy, non-regenerating muscle fibres, located in the fibre periphery under the plasma membrane (sarcolemma). Muscle fibres are bundled into fascicles by connective tissue. Each anatomical muscle is made up of a large number of fascicles again contained by denser connective tissue. As indicated above, the connective tissue ensheathing and containing muscle fibres serves the main purpose of transferring tension to the proximal and distal tendons.
Individual muscle fibres are quite homogenous in terms of structure and organelles along their length. In molecular terms, this means that the nuclei along the length of a muscle fibre produce a specific assortment of messenger RNA (mRNA), giving rise to a particular blend of proteins, defining the contractile and metabolic characteristics of the muscle fibre. The main proteins responsible for muscle contraction are actin and myosin, both of which are arranged into filaments densely packed into myofibrils. Myofibrils make up some 80 per cent of the volume of a muscle fibre in most vertebrate muscle fibres. The remaining space inside muscle fibres is taken up by sarcoplasma containing the nuclei, the sarcoplasmic reticulum involved in excitation contraction coupling and the mitochondria necessary for aerobic energy metabolism, as well as a ready store of substrates for mitochondria, such as glycogen and lipids.
The basic building block of myofibrils is the sarcomere. Sarcomeres are the contractile units of skeletal muscle fibres. They consist of a dense array of actin (thin) and myosin (thick) filaments orderly arranged between two Z-bands (Figures 1.1 and 1.2). The actin filaments of adjacent sarcomeres overlap in the Z-band, cross-linked by α-actinin, thus transferring force from one sarcomere to the next. In the middle of each sarcomere, actin and myosin filaments overlap. By addition of Ca++, sarcomeres shorten as the myosin filaments pull the actin filaments towards the sarcomere centre. The combined shortening of thousands of sarcomeres in series results in macro-scopical shortening of muscle fibres (for a schematic representation of critical muscle proteins discussed below, see Figure 1.3).
Myosin is the main protein of the thick filament. Myosin is a hexameric protein consisting of two identical myosin heavy chains (MHC) and two pairs of light chains (MLC). A single myosin filament consists of two intertwined myosin heavy chains forming a tail with the hinge region carrying the myosin head portions. The hinge region is thought to be critical to molecular movement, responsible for the movement of myosin heads during the power stroke. In the hinge region, the myosin heavy chains are associated with a regulatory and an essential myosin light chain. The strategic location of myosin light chains in the hinge portion of the myosin filament allows the regulatory light chain to stabilize the hinge region and to fine-tune the motor function of the myosin head movement (Hernandez et al., 2007). The regulatory light chain is subject to phosphorylation by myosin light chain kinase (MLCK) and may, by interaction with actin, modify force production in skeletal muscle (Hodgson et al., 2005).
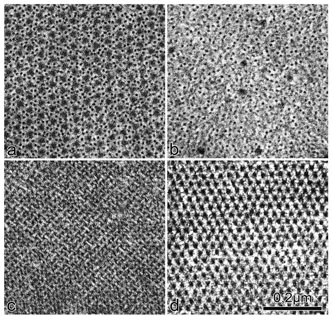
Figure 1.1 Electron microscopic image of longitudinal section through a sarcomere (arrow denotes glycogen granules)
Figure 1.2 Cross sections of a muscle fibre. Panel (a): A-band region with overlapping actin and myosin filaments. Panel (b): I-band region containing only actin. Panel (c): Z-band region showing characteristic “weaver basket” appearance. Panel (d): M-band region showing connections between myosin filaments
Energy for contraction is derived from hydrolysis of adenosine triphosphate (ATP). The ATP cleaving site is located in the myosin head portion. The activity of the myosin ATPase is responsible for the rate of ATP turnover and determines how quickly bonds between actin and myosin can be formed and broken. This means that the activity of the myosin ATPase is a major determinant of the speed of contraction. Myosin heavy chains and myosin light chains occur as multiple protein isoforms that vary in functional properties. This gives rise to skeletal muscles with vastly differing mechanical properties. Adult human muscle expresses one slow myosin heavy chain and two fast heavy chains (MHCIIA and MHCIIX). The expression of protein isoforms is malleable with development but also with exercise training, allowing for subtle modifications of mechanical behaviour as a consequence of specific mechanical requirements.
The thick filament consists of some 300 individual myosin filaments arranged in an antiparallel structure with a central bare area devoid of myosin heads. Thick filaments are arranged into a hexagonal lattice with the actin filaments occupying the space between myosin filaments (Figures 1.2a). On longitudinal sections of muscle tissue observed with electron microscopy, we can discern the I- and A-band region of the sarcomere. The central region of the A-band exhibits a fine striation pattern, the M-bands. The major proteins responsible for linking thick filaments in the M-band region are myomesin and M-protein, two proteins of similar molecular structure (Agarkova and Perriard, 2005). Myomesin dimers arranged in an antiparallel fashion are thought to be the main component of an elastic scaffold spacing thick filaments. In this function, myomesin is also responsible for restricting longitudinal misalignment of thick filaments cooperating with titin (explained later in this chapter) in maintaining sarcomeric integrity under active and passive load. Additional sarcomere stability is provided by cytoskeletal components, such as spectrin, ankyrin and obscurin, linking M-bands with M-bands of neighbouring myofibrils and with the sarcolemma. In the cross-bridge containing zone of the sarcomere, there is an additional abundant protein, myosin binding protein-C (MyBP-C). MyBP-C is arranged in a trimeric fashion around the thick filament backbone extending outwards to the actin. Its role in skeletal muscle is not well understood; it is believed to have a role in assembly and stabilization of the sarcomere and in modulating myosin-actin interaction. In cardiac muscle, mutations in the gene encoding cardiac MyBP-C are a common cause of hypertrophic cardiomyopathy (Ackermann and Kontrogianni-Konstantopoulos, 2010). In addition to the proteins mentioned above with mainly structural functions, we also find creatine-kinase located in the M-band region. Creatine-kinase buffers local ATP availability during muscle contraction by reversible exchange of high-energy phosphate bonds between phosphocreatine and ADP.
Actin is one of the major cytoskeletal proteins in eukariots. It plays an essential role in cellular processes such as cell migration, cytokinesis and vesicle transport. For the contractile activity of skeletal muscle, actin is arranged into actin filaments of similar length. The thin filament actin (F-actin) consists of two intertwined strings of globular actin molecules (G-actin). Actin filaments have a polarity, with one end (the barbed end; + end) where G-actins are added more rapidly than at the pointed end (− end). In the stable F-actin configuration, both ends of the filament are capped with the barbed end anchored in the Z-band. The globular actin subunits form a dynamic pool in muscle fibres, out of which actin monomers can be recruited to form the actin filaments. In adult skeletal muscle, this pool of G-actin constitutes a diminishing small portion of less than 1 per cent of the total actin in a myo-fibre. Filament formation in skeletal muscle is energy dependent, but the control of this process is not understood in detail. The barbed ends of sarcomeric actin filaments of adjacent sarcomeres are cross-linked and stabilized with α-actinin within the Z-band. Free actin can be incorporated into existing actin filaments without changing overall filament length; the mechanism and its control are debated.
The thin α-actin filaments, anchored in the Z-band, are by no means the only actin filaments in skeletal muscle. Over the past few years, it has become increasingly clear that the cytoskeletal actin filament system (β- and γ-actins) has a major role in myofibrillogenesis, mechanical support, ion channel function and vesicle trafficking in skeletal muscle fibres (see Kee et al., 2009). There is evidence that β-actin is important for the development and maintenance of the neuromuscular junction, in particular for the development of the postsynaptic folds and the clustering of acetylcholine receptors (AChR). γ-actin is found in the filament network around costameres (associated with the dystroglycan complex), around mitochondria and co-localizing with the Z-line. γ-actin is further suggested to play a role in the peripheral location of myonuclei and may have a role in ion channel location and operation. Cytoskeletal isoforms of tropomyosin (see below) are associated with γ-actin and have yet-to-be-determined functions for the two specialized membrane systems in skeletal muscle, the t-tubules and the sarcoplasmic reticulum (SR). In this location, and possibly also at the costamere and at the neuromuscular junction, additional actin binding cytoskeletal components, spectrin and ankyrin, function as mechanical linkers. It is further well established that the actin cytoskeleton plays a crucial role in the insulin-dependent active translocation of the glucose transporter GLUT 4 to the sarcolemma and to t-tubules. In more general terms, the actin cytoskeletal and associated proteins are held responsible for vesicle movement, tethering and docking in skeletal muscle fibres as in cells of other tissues.
Tropomyosin and troponin are well-characterized proteins that bind to the sides of the muscle actin filament. Tropomyosin stabilizes the actin filament and is involved in regulation of muscle contraction. Tropomyosin is a two-stranded helical coiled-coil molecule of 40 nm length follow...