
- 272 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This title, first published in 1987, provides an authoritative account of both the science and the politics of acid rain. Chris Park places the debates surrounding acid rain in context, and examines the full implications of scientific studies and the effects of acid rain on surface waters, soils and buildings. Evidence is drawn from around the world, including an examination of the damage in Scandinavia and Germany and the effects of acid rain in the U.K. and U.S.A. A comprehensive and relevant work, this is an important guide for students of geography, environment and sustainability and energy policy.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Acid Rain (Routledge Revivals) by Chris C. Park in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Ecology. We have over one million books available in our catalogue for you to explore.
Information
Part I
THE PROBLEM OF ACID RAIN
1 THE ACID RAIN DEBATE IN CONTEXT
‘Long to reign over us …’
this most excellent canopy, the air, look you, this brave o’erhanging firmament, this majestical roof fretted with golden fire, why, it appeareth nothing to me but a foul and pestilent congregation of vapours.
(William Shakespeare, Hamlet, II, ii, 299)
Acid rain has been called ‘an unseen plague of the industrial age’ (Anon 1984b: 6), and it is generally regarded as one of the most serious environmental problems of our times. As an issue with both scientific and political dimensions it ranks alongside important contemporary concerns like the global increase of carbon dioxide in the atmosphere, the spread of toxic chemicals in the environment, and the possible environmental consequences of nuclear war. However, it presents a somewhat unique problem in that its consequences are already evident, its adverse effects are already documented, and its impacts are very real to people living in affected areas (Whelpdale 1983: 72).
Acid rain is a widespread, serious, and costly problem that will not simply fade away in the public consciousness or cease to create environmental damage. It has been blamed for damage to trees in West Germany, death of fish in Scandinavia, and other unwanted effects (including damage to buildings and human health), and it threatens international relations between the United States and Canada, and between Britain and her European neighbours. The Organization for Economic Co-operation and Development (OECD) has estimated that acid rain costs the countries of the European Community £33–44 billion per annum (The Times, 19 November 1985, p. 16).
This book seeks to review the main arguments currently being levelled in the ‘acid rain debate’. We shall examine the general context of acid rain in this chapter, and then progress to examine the effects it is believed to have on lakes and rivers, forests and crops, humans and buildings (in Part II) and to explore possible solutions and remedies (in Part III). The diplomatic and political ingredients of the debate are considered in Part IV.
The term ‘acid rain’ refers to the dilute sulphuric and nitric acids which, many believe, are created when fossil fuels are burned in power stations, smelters, and motor vehicles, and which fall over areas long distances downwind of possible sources of the pollutants. For convenience the term ‘acid rain’ will be used throughout this book, although it has been dismissed as ‘emotive’ and ‘inadequately named’ (correspondence by Professor B.A. Thrush to The Times, 22 September 1984, p. 9). The term is a misleading short-hand version of ‘acid precipitation’, which includes the dry fallout of oxides of sulphur and nitrogen (in the form of dry gases and minute aerosols, particles that remain suspended in the atmosphere) as well as wet deposition of acids (in solution or suspension in fog, or on raindrops, snowflakes, or hail). ‘Acid rain’, as used here, includes both dry and wet deposition.
ACID RAIN AS A POLLUTANT
Pollutants can be classified into two groups (Park 1981: chapter 7). First, there are man-made materials (such as persistent synthetic chemicals like DDT), which are not part of natural environmental cycles and therefore do not readily break down when released into the environment. A sub-set of this group would include materials like nuclear waste products that are – strictly speaking – parts of natural cycles, but these cycles operate extremely slowly over long time-spans. The second group comprises materials that already exist naturally in the environment, and that natural environmental processes and cycles can cope with (or neutralize), break down, disperse and recycle, but that appear in much higher concentrations than would normally be the case. Pollutants of this type are not necessarily harmful in themselves – they create problems only when they overload natural biogeochemical cycles.
Acid rain falls in this second group because its basic ingredients (sulphur dioxide, SO2, nitrogen oxides, NOx, and ozone, O3) do appear naturally in the environment, albeit in smaller concentrations. In fact the natural acidity of rainfall provides free supplies of valuable nutrients for plant growth. Some areas (even parts of Scandinavia) with mineral-deficient soils are happy to receive the sulphur because otherwise costly artificial fertilizers would be required. It is the increased acidity experienced in recent years (which many associate with air pollution) that appears to produce serious environmental problems, and which is the true focus of the acid rain debate.
As a form of pollution, acid rain has some unusual properties. It is invisible, with no discernible taste or smell to humans. It has remained largely undetected even in areas where it has been falling for many years, because its effects on the environment are not readily noticeable in their early stages. It has no rapid, dramatic effects; it is a silent, creeping paralysis form of cumulative pollutant. Only in advanced cases are its effects sufficiently quantifiable to be convincing in any statistical sense (Elder 1983: 58).
Neither does acidification normally cause unsightly effects on the landscape, of the sort often associated with nutrient enrichment (eutrophication) with nitrate fertilizers washed from fields, where enriched lakes are draped with dense floating mats of algal blooms. In fact, few who have witnessed Scandinavia’s acidic lakes fail to be moved by the air of tranquillity offered by the clear lakes, devoid of smell or floating vegetation, with visibility increased considerably (through loss of aquatic life), and lake beds covered by white acid-tolerant mosses. Acidified lakes are reminiscent of Rachel Carson’s Silent Spring (1962), in which birds and animals died from the effects of toxic pesticides and insecticides like DDT.
THE GEOGRAPHY OF ACID RAIN
Acid rains and the oxides that create them are blown long distances by the wind, often crossing seas and national frontiers to become an invisible export. As a result, the polluted is often far removed downwind from the polluter, who may remain unconvinced that a real problem exists. The polluter may then raise serious and well-intentioned objections to any remedial measures proposed by the polluted that would impose a heavy cost on itself.
Added complexity arises from the fact that, although sulphur and nitrogen oxides are now relatively ubiquitous in industrial nations, not all areas are affected by acid rain, and different locations (even within an area) show different symptoms of acidification. As yet, the areas where acid rain impacts are noticeable have been relatively few and predictable, given the ingredients of the ‘acid rain equation’. The most seriously affected areas are shown in Figure 1.1. They tend to have a number of properties in common (La Bastille 1981: 660):
• they are concentrated in the industrialized belt of the northern hemisphere, downwind of dense concentrations of power stations, smelters, and large cities;
• they are often upland or mountainous areas, which are well watered by rain and snow;
• being well watered, they are often dissected by lakes and streams, and often covered by forest; and
• being upland, they often have thin soils and glaciated bedrock.
Many parts of Scandinavia, Canada and the Northeast United States, and northern Europe (particularly West Germany and upland Britain) share these properties, and this is why they figure so prominently in the acid rain debate. Across the Atlantic there are a number of ‘acid rain hot spots’, including Nova Scotia, the Canadian Shield around southern Ontario and Quebec, the Adirondack Mountains, Great Smoky Mountains, parts of Wisconsin and Minnesota, the Pacific Northwest USA, the Colorado Rockies, and the Pine Barrens of New Jersey (La Bastille 1981).
In contrast, there are two types of ‘safe area’, where acid rain is not a problem (at present). One comprises those areas that simply do not receive acid rain or the gaseous oxides of sulphur and nitrogen, because of the fortunes of location (away from and not downwind of possible source areas). Almost all of the southern hemisphere, the tropics and parts of the northern hemisphere are so protected (up to now). The second group comprises areas that receive acid precipitation (wet or dry) but can tolerate it. Many areas have a natural resistance (or ‘buffering capacity’ – see chapter 3) to acidification, with immunity offered by alkaline soils or limestone bedrock, which neutralize acid inputs. Other forms of buffering are offered in the Midwest United States, where alkaline dust blown from the west neutralizes acid rain before it reaches the ground (La Bastille 1981). Alkaline precipitation has been observed in Sweden in the past (pre-1960) in areas with limestone outcrops and areas where cement was manufactured (Barrett and Brodin 1955).
Figure 1.1 The global nature of acid rain in the early 1980s
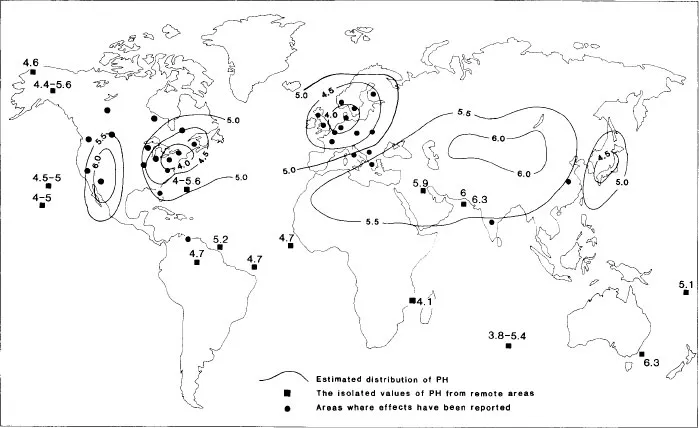
Source: estimated distribution of precipitation pH after Whelpdale (1983, Figure 1), isolated values after Galloway et al. (1982), areas where effects have been reported after Dudley, Barrett, and Baldock (1985, Figure 1).
ACID RAIN IN HISTORIC CONTEXT
Concern over acid rain may be a product of the last decade, but acid rain itself most certainly is not. It is very much a product of the industrial age. Although a natural background level of acid rain (derived from natural sources such as volcanic eruptions) has existed through the ages, it is only within the last 200 years that the widespread burning of fossil fuels has led to a dramatic (man-made) increase in emissions of sulphur and nitrogen oxides, and a corresponding increase in rainfall acidity.
There is evidence from many areas of this association in time. Some of the evidence reflects ecological changes triggered by acid precipitation in the past (which are described in greater detail in chapters 4 and 5). For example, the bogmoss (Sphagnum spp.) is very susceptible to SO2, and there are historic records of its disappearance from the Pennines at the time of the industrial revolution (Tallis 1964; Ferguson and Lee 1983). Records of changing diatom (microscopic marine or freshwater plants) populations, which reflect increasing acidity of the lake waters during the last two centuries, are preserved in lake sediments in some Scottish lochs (in Galloway, which presently receives acid rain) (Battarbee and Flower 1982; Flower and Battarbee 1983; Battarbee 1984; Flower 1984; Pennington 1984; Battarbee et al. 1985a, b). Many species of lichen are also intolerant of SO2, and lichen deserts are common in and around urban and industrial areas in Britain and elsewhere (Gilbert 1975).
Other evidence comes from studies of changing patterns of rainwater chemistry. Direct evidence of a ten-fold increase in atmospheric nitrate concentrations in North America and a five-fold increase in Europe since the turn of the century emerges in analyses of records made by Victorian scientists and kept at agricultural stations in Europe and North America (Brimblecombe and Stedman 1982). Indirect evidence is preserved in the chemical impurities locked up in the unique archive of polar snow and ice. As each new snow layer is incorporated into the ice, material from the overlying air is trapped within it as both particles and gases (in air bubbles). The precise age of different levels within the ice can be determined scientifically, and so the polar ice caps contain an invaluable record of pollution spanning up to half a million years. Recent analyses of ice cores from Greenland (Wolff and Peel 1985; also The Times, 24 May 1986) indicate that atmospheric concentrations of sulphate and nitrate were variable but low before the turn of the century (reflecting background levels of natural origin), but since 1900 they have increased exponentially – nitrates have doubled and sulphates have trebled.
Apart from providing a long record of changing air quality, these Greenland studies highlight the widespread distribution of the oxides (which are dispersed by prevailing upper air winds). Recent studies of ice cores from the Antarctic (Wolff 1986), which confirm that there has been no detectable increase in sulphates and nitrates in that part of the southern hemisphere (remote from industry and emission sources of pollutants) over the last fifty years, provide an interesting and salutory contrast.
The evidence of association in time complements the evidence of association in space between observed patterns of acid rain and man-made emissions of SO2 and NOx. This does not in itself prove cause and effect, but the associations have clearly not arisen by chance.
HERITAGE OF INTEREST
Widespread scientific interest has only started to focus on acid rain within the last decade. This reflects the growing seriousness of the problem and the related rise of acid rain as a political issue, but it is slightly surprising given over a century of research by scientists. There are four leading figures in this intellectual dynasty (Cowling 1982; La Bastille 1981: 661–2).
Robert Angus Smith, an English chemist who was Britain’s first Alkali Inspector (i.e. air pollution watchdog), was the subject’s founding father. In 1852 Smith discovered a link between the sooty skies over industrial Manchester and the acidity he found in precipitation, and twenty years later he used the term ‘acid rain’ in a 600-page book on the subject (Smith 1872; Gorha...
Table of contents
- Cover Page
- Half Title Page
- Title Page
- Copyright Page
- Original Title Page
- Original Copyright Page
- Contents
- Tables
- Figures
- Acknowledgements
- Preface
- Part I The Problem of Acid Rain
- Part II The Science of Acid Rain
- Part III The Technology of Acid Rain
- Part IV The Politics of Acid Rain
- Bibliography
- Index