
- 328 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Energy is typically regarded as understandable, despite its multiple forms of storage and transfer. Entropy, however, is an enigma, in part because of the common view that it represents disorder. That view is flawed and hides entropy's connection with energy. In fact, macroscopic matter stores internal energy, and that matter's entropy is determined by how the energy is stored. Energy and entropy are intimately linked.
Energy and Entropy: A Dynamic Duo illuminates connections between energy and entropy for students, teachers, and researchers. Conceptual understanding is emphasised where possible through examples, analogies, figures, and key points.
Features:
- Qualitative demonstration that entropy is linked to spatial and temporal energy spreading, with equilibrium corresponding to the most equitable distribution of energy, which corresponds to maximum entropy
- Analysis of energy and entropy of matter and photons, with examples ranging from rubber bands, cryogenic cooling, and incandescent lamps to Hawking radiation of black holes
- Unique coverage of numerical entropy, the 3rd law of thermodynamics, entropic force, dimensionless entropy, free energy, and fluctuations, from Maxwell's demon to Brownian ratchets, plus attempts to violate the second law of thermodynamics
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Energy and Entropy by Harvey S. Leff in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Energy. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Energy is Universal
CONTENTS
1.1Mysterious Invisible Energy
1.1.1Internal energy
1.1.2Brownian motion
1.2Caloric: A Seductive Idea
1.3Energy Transfers: Work, Heat, Mass
Work
Heat
Material transfer
1.4Imagined Systems with E = Constant
1.4.1Rigid bodies
1.4.2Frictionless surfaces
1.5Dilute Gas Model: Ideal Gas
1.6Energy Definitions, Units
1.7Energy Transformation Examples
1.1Mysterious Invisible Energy
Everything that happens in the universe entails energy. There are various forms of stored energy, namely, the energy of motion (kinetic energy), energy due to electromagnetic forces (EM energy), energy due to gravitation (gravitational energy), and energy associated with nuclear forces. Nonzero kinetic energy occurs for a moving object that travels from one place to another. It also is involved when an object spins and/or vibrates. Electromagnetic energy binds electrons to nuclei in atoms, and nuclear energy holds nuclei together.
Energy release is associated with chemical bonds when the product compounds in a chemical reaction have lower total energy. Examples include combustion of coal, natural gas, petroleum, wood, and candle wax. As I shall explain, the stored energy is negative, and energy release is the result of even more negative energies stored in the combustion products, primarily CO2 (carbon dioxide) and H2O (water).
Invisible energy is ubiquitous in the universe. It enables plants to grow and animals to live. It resides in solids, liquids, gases, plasmas, and in fact just about everything. Outside Earth, energy drives the birth, life, and death of each star, which stores and converts enormous amounts of energy into light, heat, neutrinos and other particles. Energy transformations run the universe.
Thermodynamics is the science devoted to the study of energy and its transformations within and between macroscopic bodies. These transformations typically entail spatial redistributions of energy. The term macroscopic connotes virtually any matter that has a very large number of molecules. Typically this number is of order 1020 or greater. Examples include a human hair, speck of dust, paper clip, horseshoe magnet, human body, the air in a room, an automobile engine, multi-story building, planet, star, galaxy, and even the whole universe.
Humans’ digestive systems transform energy derived from their food and the oxygen breathed in, enabling their hearts to pump blood, their muscles to work, and their lungs to continually take in oxygenated air and expel carbon dioxide. Energy is delivered to our homes via electricity and (typically) fossil fuels, along with the oxygen in the air. That energy is used to perform functions that keep us warm in winter and cool in summer, keep foods cold, cook meals, run our computers, power our kitchen appliances and TV, and generally make our lives more comfortable.
Thermodynamics is extraordinarily important, not just to humans, but to all living things. All of these depend on a continual flow of energy to them from nutrients and oxygen needed to digest them, and solar radiation to sustain their lives, and from them as they dump energy to their surroundings.
1.1.1Internal energy
Gaining an understanding of energy stored within matter is a fundamental goal of this book. Students of beginning physics learn about the kinetic energy of non-existent point-like particles (that occupy zero volume) and also about kinetic energy for objects such as disks and spheres, which can move from place to place and also rotate.
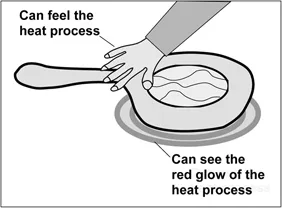
Typically there is very little, if any, attention given to the energy stored within materials. For example, the energy stored by a pot of water increases when it is heated on a stove top. We cannot see that energy, but we are aware that the object gets hotter. We can even feel that it is hot by bringing our hand near the pot. Although we do not seem to be touching anything, we sense warmth. What we feel is the effect of invisible energy, infrared radiation being emitted from the pot and traveling to our skin. Further, an electric stove’s heating coil gets so hot that it glows, as illustrated in Fig.1.1. In that case, our eyes can detect that there is energy being emitted by the red-hot coil. If the water were allowed to boil away, the pot itself might begin to glow!
Key Point 1.1 Macroscopic bodies store internal energy, which is denoted here by E. Suppose a system has zero net energy flow through its boundary, and unchanging pressure, temperature and any other measurable variables. This quiescent state is called thermodynamic equilibrium. In such an equilibrium state, the internal energy typically can be written as E(T, V, N), a function of the system’s temperature T, volume V and number of molecules N.
When a liquid like water is heated, its energy increases and some of the water molecules gain sufficient kinetic energy to break out of the liquid, to the air above it. At each temperature, the equilibrium water vapour pressure has a well-defined value. When the water temperature reaches 100 °C, its vapour pressure equals the (assumed) normal atmospheric ...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Contents
- Preface
- Acknowledgments
- Chapter 1: Energy is Universal
- Chapter 2: Energy is Not Enough
- Chapter 3: Entropy: Energy’s Needed Partner
- Chapter 4: Gases, Solids, Polymers
- Chapter 5: Radiation & Photons
- Chapter 6: Numerical Entropy
- Chapter 7: Language & Philosophy of Thermodynamics
- Chapter 8: Working, Heating, Cooling
- Chapter 9: Sanctity of the 2nd Law of Thermodynamics
- Chapter 10: Reflections & Extensions
- Chapter 11: Appendices: Mathematical Identities
- Subject Index
- Author Index