
eBook - ePub
Lightweight Polymer Composite Structures
Design and Manufacturing Techniques
- 394 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Lightweight Polymer Composite Structures
Design and Manufacturing Techniques
About this book
This book provides a comprehensive account of developments in the area of lightweight polymer composites. It encompasses design and manufacturing methods for the lightweight polymer structures, various techniques, and a broad spectrum of applications. The book highlights fundamental research in lightweight polymer structures and integrates various aspects from synthesis to applications of these materials.
Features
- Serves as a one stop reference with contributions from leading researchers from industry, academy, government, and private research institutions across the globe
- Explores all important aspects of lightweight polymer composite structures
- Offers an update of concepts, advancements, challenges, and application of lightweight structures
Current status, trends, future directions, and opportunities are discussed, making it friendly for both new and experienced researchers.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 Lightweight Graphene Composite Materials
Akarsh Verma, Naman Jain, Avinash Parashar, Vinay K. Singh, M. R. Sanjay, and Suchart Siengchin
Contents
1.1 Introduction
1.1.1 Synthesis of Graphene
1.1.2 Properties of Graphene
1.1.3 Graphene-Based Polymer Composites
1.2 Large-Scale Production of Graphene-Based Composite Materials
1.3 Modeling and Simulation of Graphene-Based Lightweight Composite Materials
1.4 Advanced Graphene-Based Lightweight Composite Materials
Acknowledgment
Conflicts of Interest
References
1.1 Introduction
1.1.1 Synthesis of Graphene
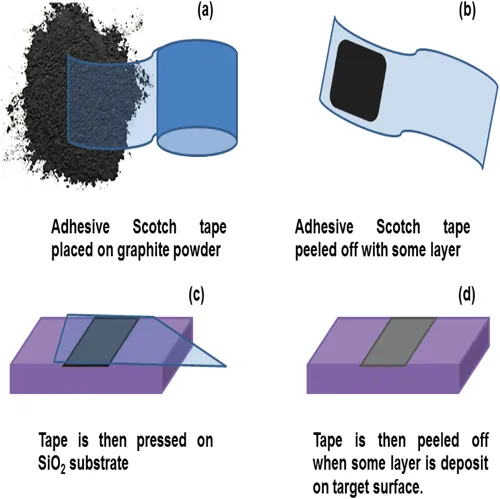
Micromechanical exploitation of graphene from the graphite is one of the simplest and oldest methods to extract graphene [1–4], as shown in Figure 1.1. In 2005, Novoselov et al. [5] extracted the two-dimensional (2D) crystallites by rubbing graphite crystal against another surface like drawing chalk on the blackboard; but, only some of the amount of flakes were present. In micromechanical exploitation, graphene 2D layers are peeled off by scotch tape, and after that deposited on SiO2. But major disadvantages of this method are its non-scalability and uneven production in a small area.
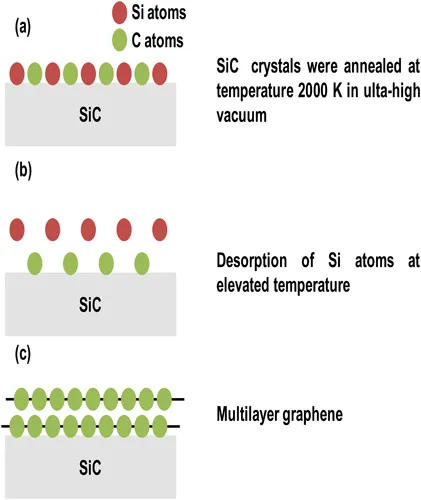
Epitaxial growth is another technique used for the synthesis of graphene [6, 7], as shown in Figure 1.2. In this technique, SiC crystals are annealed at a high temperature of about 2000 K in ultra-high vacuum. At this elevated temperature, silicon desorption occurs from the uppermost surface of SiC, resulting in yielding of multi-layer graphene. In 2004, Berger et al. [6] fabricated the graphene ultrathin films by thermal desorption of Si from 6H-SiC single crystal face. After, the hydrogen etching of the surface was done, and then samples were heated to 1000°C to remove the oxides. Graphene layers were controlled by limiting heat treatment either by temperature or time. The epitaxial growth technique yielded larger area compared to the exfoliation method, but still the size is not large enough for those required in electronic applications.
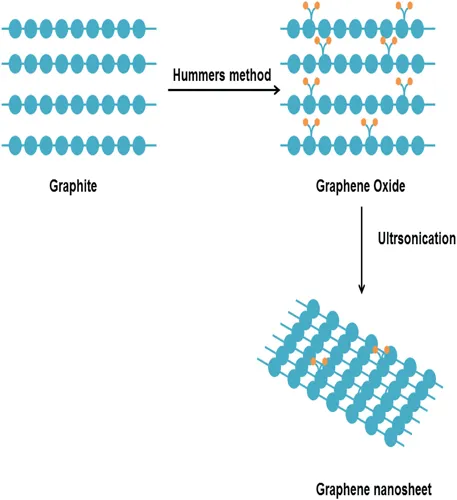
Researchers also employed the wet chemical approach to synthesize graphene from graphene oxide [8–10], as shown in Figure 1.3. In this process, graphite is first converted into graphite oxide by nitric and sulphuric acid treatment. Then, rapid evaporation of intercalant is done at high temperatures, followed by a ball milling process. Stankovich et al. [8] in 2007 prepared graphene by the wet chemical approach. Firstly, graphene oxide (GO) was prepared by the hummers method from pure nature graphite. Then 10 mg of GO was added into 100 ml water, which yielded in yellow-brown inhomogeneous dispersion. Ultra-sonication of dispersion was done to clear the particulate matter. After that, hydrazine hydrate was added and the resulting solution was heated to 100°C for 24 hours under condenser, which reduced GO to a black solid. This method is more versatile than the above two methods, but it also has certain limitations such as poor control of number of layers and partially oxidized graphene is obtained that may alter the electronic and mechanical properties.
The most effective technique to synthesize the graphene sheet is the chemical vapor deposition (CVD) approach [11–15], as shown in Figure 1.4. In this technique, hydrocarbon gases such as methane and hydrogen are heated to a 1000°C temperature into a furnace. A thin layer of transition metallic which is deposit to substrate is used to catalyze the decomposition gas. The metallic layer gradually gets absorbed or diffused, dissociating carbon atoms depending upon the metal. Many researchers have used different metallic catalysts such as Ru, Ir, Pd, Ni, Cu, etc. Mainly, two types of mechanisms occur in the CVD process:
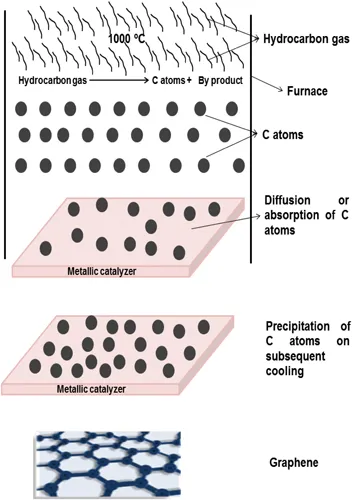
- (1) The decomposed carbon atoms are absorbed by the metallic catalyzer and then upon subsequent cooling (with decrease in temperature solubility of carbon decreases) carbon atoms precipitate on the surface of metal to form graphene. This mechanism occurs in those metals which have strong interaction with carbon atoms e.g., Ni.
- (2) The decomposed carbon atoms remain or diffuse into metallic catalyzer surface and are then incorporated into graphene. This mechanism occurs in those metals which do not form carbide such as Cu.
1.1.2 Properties of Graphene
Graphene is made up of two-dimensional hexagonal rings like honeycomb lattice with carbon atoms. In graphene, carbon atoms are covalently bonded with each other with sp2 hybridization state. Each carbon atom in graphene is bonded with three neighboring carbon atoms, although it has the tendency to make a bond with the fourth atom also. A molecular model of graphene can be visualized as the planar benzenoid hexagonal rings such that peripheral hydrogen atoms are saturated. When these graphene layers are stacked up to form a three-dimensional structure, it is known as graphite (which is commonly used in day-to-day life such as pencils, batteries, and more). Each carbon atom of graphene lies on the surface, due to which there is more interaction with the surrounding atoms. High surface area to volume ratio, elevated stiffness, and distinct electrical and thermal properties of graphene have gained researcher interest as well as their potential application to the area of nanotechnology. At room temperature, the mobility of electrons in graphene is very high due to its two-dimensional structure, that further results in high electrical and thermal conductivity; but on the other hand, there is no band gap in graphene, which restricts its application in the electronics sector. Nowadays, researchers are working to overcome the above problem in many ways such as applying the electrical field or by doping. As per the study of Feng et al., [16] energy gap and binding energy of graphene are strongly affected by the size, compared with the stacking sequence and number of layers. Broad energy gaps in the range 1–2.5 eV have been obtained by π-π interaction between graphene finite size sheets. Another way to open the energy gap is to pattern the graphene into a narrow ribbon in which carriers are confined into a quasi-one-dimensional system and energy gap varies inversely with the ribbon width [17]. Another alternative may be hydrogenation of the graphene. In 2010, Samarakoon and Wang [18] applied bias voltage across the hydrogenated graphene sheet, which resulted in continuous tuning of energy gap. In 2009, Pereira et al. [19] studied the effect of mechanical strain on the electronic structure of graphene. They observed that to generate a gap, minimum threshold must be applied along the specific lattice direction. Gui et al. [20] also investigated the graphene structure under planer strain. Their results found that zero band gaps were obtained with applied symmetrical strain, while asymmetrical strain results in opening of Fermi level energy gap. When strain was applied along the C-C bonds, maximum energy gap of about 0.486 eV was obtained, whereas the maximum gap of about 0.170 eV was obtained when the strain was applied perpendicular to the C-C bonds.
In parallel to the electrical properties of graphene, graphene-based composites also have fascinating mechanical properties. The Young’s modulus E3D on the material can be calculated in terms of shear energy γs by the simple equation where V 0 is the volume of material. Whereas in graphene, in-plane stiffness is defined as compared to E 3D, which is calculated by the equation
Table of contents
- Cover
- Half-Title
- Title
- Copyright
- Contents
- Preface
- Editor Biographies
- Contributors
- Chapter 1 Lightweight Graphene Composite Materials
- Chapter 2 Conventional Processing of Polymer Matrix Composites
- Chapter 3 Biodegradable and Biocompatible Polymer Composite: Biomedical Applications and Bioimplants
- Chapter 4 Lightweight Polymer Composites from Wood Flour, Metals, Alloys, Metallic Fibers, Ceramics
- Chapter 5 Lightweight Composite Materials in Transport Structures
- Chapter 6 Hybrid Thermoplastic and Thermosetting Composites
- Chapter 7 Design and Modeling of Lightweight Polymer Composite Structures
- Chapter 8 Smart Lightweight Polymer Composites
- Chapter 9 Carbon Fiber Reinforced Thermoplastics and Thermosetting Composites
- Chapter 10 Glass Fiber Thermoset and Thermoplastic Composites
- Chapter 11 Inorganic Nanofillers-Based Thermoplastic and Thermosetting Composites
- Chapter 12 Applications of Thermoplastic and Thermosetting Polymer Composites
- Chapter 13 Life Cycle Assessment of Thermoplastic and Thermosetting Composites
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Lightweight Polymer Composite Structures by Sanjay Mavinkere Rangappa,Jyotishkumar Parameswaranpillai,Suchart Siengchin,Lothar Kroll in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Civil Engineering. We have over one million books available in our catalogue for you to explore.