1.1 Introduction
During the twentieth century chemistry changed the way we live forever. Perhaps the greatest perceived benefits, to the general public, have come from the pharmaceuticals industry with developments of painkillers, antibiotics, heart drugs and targeted cancer drugs. However, it is difficult to think of an important facet of modern life that has not been transformed by products of the chemical and related industries, for example.
- Transportation – production of gasoline and diesel from petroleum and more recently crops, fuel additives for greater efficiency and reduced emissions, catalytic converters, plastics to reduce vehicle weight and improve energy efficiency.
- Clothing – man-made fibres such as rayon and nylon, dyes, waterproofing and other surface finishing chemicals.
- Sport – advanced composite materials for tennis and squash rackets, all-weather surfaces, textiles that let the body breathe and reduce wind resistance.
- Safety – lightweight polycarbonate cycle helmets, fire-retardant furniture, air bags.
- Food – refrigerants, packaging, containers and wraps, food processing aids and preservatives.
- Health – chlorine for clean water supplies, blood bags, internal stitches that dissolve, anaesthetics, disinfectants, vaccines, dental fillings, artificial joints, contact lenses, contraceptives.
- Office – photocopying toner, inks, printed circuit boards, liquid crystal displays.
- Home – material and dyes for carpets, plastics for TVs, and mobile phones, CDs, paints, detergents and self-cleaning windows.
- Farming – fertilisers, pesticides.
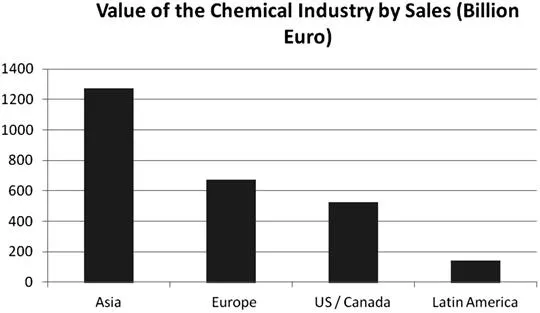
The value of the chemical industry is over 3000 billion Euro (Figure 1.1) with Asian production and China in particular accounting for most of this. In Europe, 1.3 million people are directly employed by the industry, and around 4 million including the supply chain and service sector.
Figure 1.1 Value of the global chemical industry.
In many countries, however, the chemical industry is often viewed, by the general public, as causing more harm than good. There are several reasons for this including general ignorance of the end use and value of the industry’s products, since the chemical industry rarely sells to the end consumer. However, a major reason is that the industry is perceived as being polluting and causing significant environmental damage. Although a very safe industry in general, well-publicised disasters, such as Bhopal and Deepwater Horizon in the oil sector, causing both environmental damage and loss of life have led to this generic view. As well as specific disasters, general pollution that came to the public’s attention in the 1960s and 1970s through eutrophication, foaming rivers, the discovery of persistent organic pollutants, and the famous ‘burning’ Cuyahoga river, have all played a part in formulating this view of the chemical industry.
Chemists and engineers engaged in development of chemical products and processes have never set out to cause damage to the environment or human health. Undoubtedly, risk taking and the pressures to meet production targets and reduce cost have played their part but many early incidents occurred largely through a lack of knowledge. In particular, the longer-term effects of products entering the environment were not widely recognised until relatively recently. The challenge for the chemical industry in the twenty-first century is to continue to provide the benefits we have come to rely on, in an economically viable manner, but without the adverse environmental side effects. This can be achieved through development of more environmentally benign products using less hazardous processes and raw materials. With global warming being accepted as the biggest environmental challenge we face, the chemical industry must also develop more energy-efficient processes and reduce its reliance on fossil fuels. Nevertheless, the chemical industry is also a solution provider for climate change with 3 tonnes of CO2 being saved in downstream use for every tonne of CO2 used in manufacture. In particular, products such as fuel additives and insulation play a huge role in helping move towards a lower-carbon society.
1.2 Sustainable Development and Green Chemistry
Current thinking on sustainable development came out of a United Nations Commission on Environment and Development in 1987 (Bruntland Commission), which defined sustainable development as:
“…meeting the needs of the present without compromising the ability of future generations to meet their own needs.”
Although the ideals on which sustainable development is based are not new, indeed Thomas Jefferson made similar comments in 1789, the Bruntland commission did catalyse the sustainability debate. Since 1987 governments, NGOs, society in general and industry sectors have considered what sustainable development really means and how best to start to achieve it from their own standpoint. Issues that will have a significant impact on how the move towards sustainability is approached, include time scale, likely future technology developments, and population forecasts. Two of the key aspects of sustainable development from a chemicals and energy perspective are: how fast should we use up fossil fuels and how much ‘waste’ or pollution can we safely release to the environment? There are no agreed answers to these questions but there is general agreement to develop more renewable forms of energy and to reduce pollution.
The Natural Step, an international movement, started in Sweden, dedicated to helping society reduce its impact on the environment, has developed four system conditions for sustainability:
- (a) Materials from the earth’s crust (e.g. heavy metals) must not systematically increase in nature.
- (b) Persistent substances produced by society (e.g. DDT, CFCs) must not systematically increase.
- (c) The physical basis for the earth’s productive natural cycles must not be systematically deteriorated.
- (d) There must be fair and efficient use of resources with respect to meeting human needs.
This approach recognises that the earth does have a natural capacity for dealing with much of the waste and pollution that society generates, it is only when that capacity is exceeded that we become unsustainable.
During the early 1990s the US Environmental Protection Agency (EPA) coined the phrase Green Chemistry ‘To promote innovative chemical technologies that reduce or eliminate the use or generation of hazardous substances in the design, manufacture and use of chemical products.’ Over the last 20 years Green Chemistry has gradually become recognised as both a culture and a methodology for achieving sustainability. Green Chemistry is not a new branch of chemistry but an approach to carrying out chemistry and engineering in a sustainable manner.
The 12 Principles of Green Chemistry (Box 1.1) help show how this can be achieved.
Box 1.1 The 12 principles of Green Chemistry.
Reproduced from P. C. Anastas and J. C. Warner “Green Chemistry Theory and Practice”, Oxford University Press, New York, 1998 by permission of Oxford University Press.
1. Prevention
It is better to prevent waste than to treat or clean up waste after it has been created.
2. Atom economy
Synthetic methods should be designed to maximise the incorporation of all materials used in the process into the final product.
3. Less hazardous chemical synthesis
Wherever practicable, synthetic methods should be designed to use and generate substances that possess little or no toxicity to people or the environment.
4. Designing safer chemicals
Chemical products should be designed to effect their desired function while minimising their toxicity.
5. Safer solvents and auxiliaries
The use of auxiliary substances (e.g. solvents or separation agents) should be made unnecessary whenever possible and innocuous when used.
6. Design for energy efficiency
Energy requirements of chemical processes should be recognised for their environmental and economic impacts and should be minimised. If possible, synthetic methods should be conducted at ambient temperature and pressure.
7. Use of renewable feedstocks
A raw material or feedstock should be renewable rather than depleting whenever technically and ...