
eBook - ePub
Fundamentals of Perovskite Oxides
Synthesis, Structure, Properties and Applications
- 350 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Fundamentals of Perovskite Oxides
Synthesis, Structure, Properties and Applications
About this book
This textbook entitled Fundamentals of Perovskite Oxides: Synthesis, Structure, Properties and Applications summarizes the structure, synthesis routes, and potential applications of perovskite oxide materials. Since these perovskite-type ceramic materials offer opportunities in a wide range of fields of science and engineering, the chapters are broadly organized into four sections of perovskite-type oxide materials and technology.
-
- Covers recent developments in perovskite oxides
-
- Serves as a quick reference of perovskite oxides information
-
- Describes novel synthesis routes for nanostructured perovskites
-
- Discusses comprehensive details for various crystal structures, synthesis methods, properties, and applications
-
- Applies to academic education, scientific research, and industrial R&D for materials research in real-world applications like bioengineering, catalysis, energy conversion, energy storage, environmental engineering, and data storage and sensing
This book serves as a handy and practical guideline suitable for students, engineers, and researchers working with advanced ceramic materials.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Fundamentals of Perovskite Oxides by Gibin George,Sivasankara Rao Ede,Zhiping Luo in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemical & Biochemical Engineering. We have over one million books available in our catalogue for you to explore.
Information
1 Introduction to Perovskites
Perovskites have been an important class of materials, exhibiting unusual promising functionalities in various transport and physical properties over other traditional ceramic or composite materials. They have received extensive attention more recently in the fields of materials science, physics, chemistry, geology, and engineering. In this chapter, we give a brief introduction to perovskites regarding their history, formation, and classification.
1.1 History of Perovskites
The term perovskite originated from the mineral perovskite (CaTiO3) named after the Russian mineralogist Mr. Lev Alekseyevich von Perovski (1792–1856) (Liu et al. 2017). A photo of the CaTiO3 mineral is shown in Figure 1.1. Nowadays, perovskites represent a wide class of materials with similar or derived crystal structure as that of CaTiO3 with a general formula ABX3, as shown in Figure 1.2. In the structure of perovskites, A and B are cations and X is the anion, and A cations are larger than B cations (Figure 1.2a). Six X anions form an octahedron covering the smaller cation B (Figure 1.2b). Therefore, this perovskite lattice is formed by such apex-connected octahedra with A cations between them (Figure 1.2c).

FIGURE 1.1 A photo of CaTiO3 mineral. (From https://en.wikipedia.org/wiki/Perovskite.)
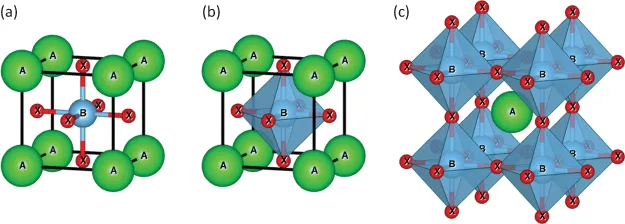
FIGURE 1.2 Perovskite ABX3 lattice. (a) Large A and small B cations, with X anions; (b) octahedron by X anions; (c) octahedra lattice.
Often A-site ion provides the structural integrity to the perovskite structure, and B-site ion determines the properties. CaTiO3 crystallizes into an orthorhombic structure; however, the ideal perovskite structure belongs to the cubic space group (No. 221). Apart from CaTiO3, many naturally existing minerals adopt a perovskite structure, for instance, ilmenite (FeTiO3), MgSiO3, etc. The silicate perovskites containing Mg, Ca, and Fe are assumed as the most abundant solid phase in the earth’s lower mantle at 670–2,900 km from the surface. Moreover, MgSiO3 is stable above 23 GPa (Zhang et al. 2014), and the same is believed to constitute the big planets other than Mars (Umemoto et al. 2006). Many perovskite materials exhibit exceptional properties such as high absorption coefficient, long-range ambipolar charge transport, low exciton-binding energy, high dielectric constant, and ferroelectric properties.
BaTiO3 is the first manmade perovskite synthesized during World War II in 1941; however, it’s naturally existing counterpart benitoite is an extremely rare mineral. BaTiO3 was used as a ferroelectric and piezoelectric material for a wide range of applications (Eshita et al. 2014). Its application as a piezoelectric material is later replaced by lead zirconate titanate Pb(Zr, Ti)O3, another important perovskite. Similarly, SrTiO3 is the first insulator, and the first oxide reported as superconductive below 0.35 K. Even though SrTiO3 was synthesized in the 1950s, its natural counterpart was discovered in 1982. SrTiO3’s first-ever use was as a simulant for diamond due to its resemblance to a diamond, but the softness and high cost resulted in their replacement with other simulants such as yttrium aluminum garnet (YAG), gadolinium gallium garnet (GGG), and cubic zirconia.
1.2 Formation of Perovskites
Since the discovery of BaTiO3, a large number of perovskites with general formula ABO3 have been synthesized and studied for their unique properties pertaining to the presence of two different cations in their lattice. In general, the new perovskite structured materials possess a wide range of properties such as optical, magnetic, thermoelectric, piezoelectric, photochemical, thermochromic, electrochromic, and electrochemical, which are not observed in their ancestors. Besides the general physical and chemical properties, these materials are extensively studied for their applicability as electrode materials for energy storage and scavenging, e.g., LaMnO3, Ba0.5Sr0.5Co0.8Fe0.2O3, etc. Despite the low-cost and ea...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- List of Figures
- List of Tables
- List of Symbols
- Preface
- Authors
- Chapter 1 Introduction to Perovskites
- Chapter 2 Synthesis of Perovskite Oxides
- Chapter 3 Crystal Structure of Simple Perovskites
- Chapter 4 Structural Variants of Perovskite Oxides
- Chapter 5 Magnetic Properties of Perovskite Oxides
- Chapter 6 Electronic Properties of Perovskite Oxides
- Chapter 7 Diffusion, Thermal, and Optical Properties of Perovskites
- Chapter 8 Applications of Perovskite Oxides
- Appendix A1: Examples of Single Perovskites with the Structural Lattice Parameters
- Index