
- 176 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
The EPA has established regulations which classify four types of disinfection byproducts - TTHMs, haloacetic acids, bromate, and chlorite - and requires public water systems limit these byproducts to specific levels. Most of the information required to comply with these standards is either scattered throughout the literature or derived from confere
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Disinfection Byproducts in Drinking Water by Yuefeng Xie in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Environmental Management. We have over one million books available in our catalogue for you to explore.
Information
1
Disinfection Byproducts
OBJECTIVES
This chapter gives a brief overview of various disinfection byproducts (DBPs), including their formation and occurrence in drinking water. The chapter also provides an extensive discussion on the nomenclature and molecular structures for various groups of DBPs. With these molecular structures, this chapter will help water professionals to better understand the behavior of DBPs during their analyses and control.
This chapter is written for people who are new in the DBP field. It clearly identifies the basic chemical formula of all major DBPs. In comparison to other chapters, especially Xie’s Bar Theory for Water Coagulation in Chapter 10, this chapter is theoretical but essential. A good understanding of the nomenclature and molecular structures for various groups of DBPs lays a solid foundation for proficient DBP analysis and control.
NOMENCLATURE
DBP disinfection byproduct
D-DBP disinfectants and disinfection byproducts
E-MX (E)-2-chloro-3-(dichloromethyl)-4-oxobutenoic acid
HAA haloacetic acid
ICR Information Collection Rule
MX 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone
NDMA N-Nitrosodimethylamine
NOM natural organic matter
THM trihalomethanes
TOX total organic halide
U.S. EPA United States Environmental Protection Agency
Disinfection byproduct (DBP) is a term used to describe a group of organic and inorganic compounds formed during water disinfection. These byproducts are formed by the reactions between disinfectants and natural organic matter (NOM) or inorganic substances in water. For oxidation processes such as ozonation, their byproducts are also referred to as DBPs even though the main purpose of the processes is oxidation. Because of their potential health risks, currently, four groups of DBPs are regulated under the United States Environmental Protection Agency (U.S. EPA) Stage 1 Disinfectants and Disinfection Byproducts (D-DBP) Rule. These four groups are trihalomethanes (THMs), haloacetic acids (HAAs), chlorite, and bromate. Additional DBPs were also monitored under the U.S. EPA Information Collection Rule (ICR). They are halopropanones, haloacetonitriles, trichloroacetaldehyde hydrate, trichloronitromethane, and cyanogen chloride. Ozone, a powerful disinfectant and oxidant, has been increasingly used in drinking water treatment. In addition to bromate, three types of organic DBPs, including aldehydes, ketoacids, and carboxylic acids are commonly detected in ozonated drinking water.
Although there are many DBPs detected in drinking water, based on their molecular structures, formation, and chemical properties, these DBPs are often categorized into the following groups.
1.1 TRIHALOMETHANES
Trichloromethane, or chloroform, was the first DBP identified in chlorinated water. Trihalomethanes are commonly abbreviated as THMs. There are four common THMs. Their names, chemical formula, and common acronyms are listed in Table 1.1.
As illustrated in Figure 1.1, a methane molecule contains four hydrogen atoms. By replacing three hydrogen atoms with halogen atoms, including chlorine and bromine, a total of four THMs are obtained, as shown in Figure 1.2.
With three chlorine atoms, the THM is trichloromethane (CHCl3). Trichloromethane is also commonly called chloroform. Bromodichloromethane (CHBrCl2) contains one bromine atom and two chlorine atoms and chlorodibromomethane (CHBr2Cl) contains one chlorine atom and two bromine atoms. Tribromomethane (CHBr3) contains three bromine atoms and is also commonly called bromoform.
TABLE 1.1
Names and Acronyms for THMs
Names and Acronyms for THMs
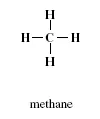
FIGURE 1.1 Molecular structure of methane.
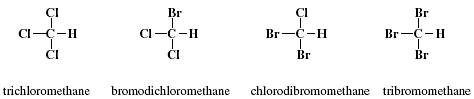
FIGURE 1.2 Molecular structures of four THMs.
Although chloroform and other THMs are illustrated as chlorinated or brominated methanes, the formation of THMs in chlorinated water is not due to reactions between chlorine and methane or chlorinated methanes. Instead, it is due to a complex reaction between chlorine and NOM, including humic or fulvic substances, as shown in Equation 1.1. The formation of brominated THMs is due to the bromide ion in chlorinated water.
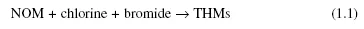
In addition to four bromo- and chloro-THMs, iodo-THMs have also been identified in chlorinated water containing iodide, especially in sea water. The introduction of iodine significantly increases the number of THMs. Considering the different combinations of chlorine, bromine, and iodine, theoretically there could be a total of 27 THMs.
1.2 HALOACETIC ACIDS
Haloacetic acids are another major group of DBPs, following THMs, in chlorinated drinking water. Haloacetic acids are commonly abbreviated as HAAs. There are nine common HAAs. Their names, chemical formula, and common acronyms are listed in Table 1.2.
As shown in Figure 1.3, an acetic acid molecule contains three hydrogen atoms at the alpha position, or the position next to the COOH group.
By replacing the hydrogen atoms with halogen atoms, partially or completely, a total of nine HAAs are obtained. Historically, haloacetic acids have been categorized as one single group. However, there are actually three groups of haloacetic acids. With one halogen the HAAs are called monohaloacetic acids (CH2XCOOH). With two halogens the HAAs are called dihaloacetic acids (CHX2COOH). With three halogens the HAAs are called trihaloacetic acids (CX3COOH). It is important to remember that there are three different types of HAAs because their formation and chemical and biological properties are significantly different.
1.2.1 MONOHALOACETIC ACIDS
With one chlorine or bromine, there are two monohaloacetic acids. They are monochloroacetic acid (CH2ClCOOH) and monobromoacetic acid (CH2BrCOOH), as shown in Figure 1.4.
TABLE 1.2
Names and Acronyms for HAAs
Names and Acronyms for HAAs
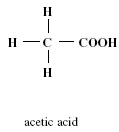
FIGURE 1.3 Molecular structure of acetic acid.
1.2.2. DIHALOACETIC ACIDS
Dihaloacetic acids contain two halogens, chlorine and/or bromine, and there are three possible combinations. They are dichloroacetic acid (CHCl2COOH), bromochloroacetic acid (CHBrClCOOH), and dibromoacetic acid (CHBr2COOH), as shown in Figure 1.5.
1.2.3 TRIHALOACETIC ACIDS
Trihaloacetic acids contain three halogens of either chlorine, bromine, or a combination of the two and being similar to THMs, there are four possible combinations. They are trichloroacetic acid (CHCl3COOH), bromodichloroacetic acid (CHBrCl2COOH), chlorodibromoacetic acid (CHBr2ClCOOH), and tribromoacetic acid (CHBr3COOH), as shown in Figure 1.6.
Again, the formation of haloacetic acids in chlorinated water is not due to the reaction between acetic acid and chlorine. HAAs are formed by the reaction between natural organic matter and chlorine, as shown in Equation 1.2.

FIGURE 1.4 Molecular structures of two monohaloacetic acids.
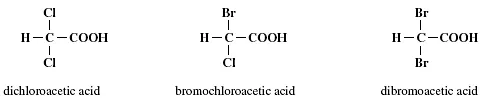
FIGURE 1.5 Molecular structures of three dihaloacetic acids.
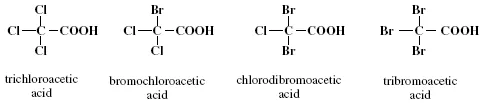
FIGURE 1.6 Molecular structures of four trihaloacetic acids.
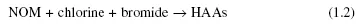
Further chlorination of monohaloacetic acids and dihaloacetic acids is not the formation mechanism for dihaloacetic acids and trihaloacetic acids in chlorinated water. They are formed through different precursors, as discussed in Chapter 2.
1.3 INORGANIC DISINFECTION BYPRODUCTS
Two inorganic disinfection byproducts are currently regulated under the Stage 1 D-DBP Rule. They are chlorite (ClO2 –) and bromate (BrO3–).
Chlorite is a common DBP found in water treated with chlorine dioxide. The formation of chlorite is due to the degradation of chlorine dioxide, which is an alternative oxidant and disinfectant for drinking water treatment. In the presence of natural organic matter or other reducing agents, chlorine dioxide is reduced to chlorite as shown in Equation 1.3.

Bromate is a common DBP found in ozonated water containing inorganic bromide. The formation of bromate is due to the ozonation of the bromide ion in water, as shown in Equation 1.4.

In actuality, the formation of chlorite and bromate in water involves many complex reactions. Further discussion of this topic/subject is provided in Chapter ...
Table of contents
- Cover Page
- Title Page
- Copyright Page
- Dedication
- Preface
- The Author
- Prelude
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10