
- 576 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Phenolics in Food and Nutraceuticals
About this book
Phenolics in Food and Nutraceuticals is the first single-source compendium of essential information concerning food phenolics. This unique book reports the classification and nomenclature of phenolics, their occurrence in food and nutraceuticals, chemistry and applications, and nutritional and health effects. In addition, it describes antioxidant a
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Phenolics in Food and Nutraceuticals by Fereidoon Shahidi,Marian Naczk in PDF and/or ePUB format, as well as other popular books in Ciencias físicas & Química. We have over one million books available in our catalogue for you to explore.
Information
1
Biosynthesis, Classification, and
Nomenclature of Phenolics in Food and
Nutraceuticals
Phenolic compounds in food and nutraceuticals originate from one of the main classes of secondary metabolites in plants derived from phenylalanine and, to a lesser extent in some plants, also from tyrosine (Figure 1.1) (van Sumere, 1989; Shahidi, 2000, 2002). Chemically, phenolics can be defined as substances possessing an aromatic ring bearing one or more hydroxyl groups, including their functional derivatives. Their occurrence in animal tissues and nonplant materials is generally due to the ingestion of plant foods. Synthetic phenolics may also enter the food system via their intentional incorporation in order to prevent oxidation of their lipid components.
Plants and foods contain a large variety of phenolic derivatives including simple phenols, phenylpropanoids, benzoic acid derivatives, flavonoids, stilbenes, tannins, lignans and lignins. Together with long-chain carboxylic acids, phenolics are also components of suberin and cutin. These rather varied substances are essential for the growth and reproduction of plants and also act as antifeedant and antipathogens (Butler, 1992). The contribution of phenolics to the pigmentation of plant foods is also well recognized. In addition, phenolics function as antibiotics, natural pesticides, signal substances for establishment of symbiosis with rhizobia, attractants for pollinators, protective agents against ultraviolet (UV) light, insulating materials to make cell walls impermeable to gas and water and as structural materials to give plants stability.
Many properties of plant products are associated with the presence, type and content of their phenolic compounds. The astringency of foods, the beneficial health effects of certain phenolics or their potential antinutritional properties when present in large quantities are significant to producers and consumers of foods. Furthermore, anthocyanins are distributed widely in foods, especially in fruits, and also in floral tissues (Harborne and Williams, 2001). They may also be used as nutraceuticals in the dried and powderized form from certain fruit or fruit by-product sources. These anthocyanins are responsible for the red, blue, violet and purple colors of most plant species and their fruits and products. For example, after their extraction, colorants produced from the skin of grapes may be used by the food industry (Francis, 1993). Methods of analysis of different classes of phenolics and polyphenolics have recently appeared (Hurst, 2002).
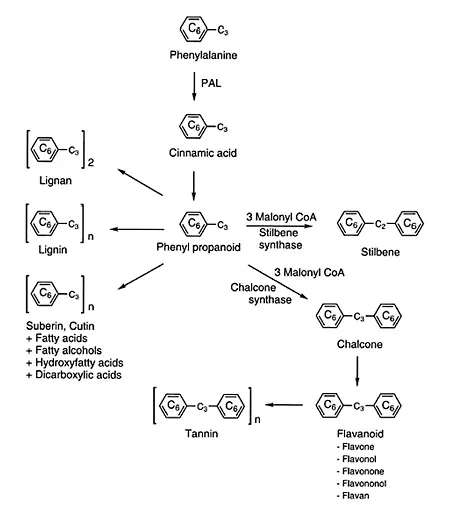
FIGURE 1.1 Production of phenylpropanoids, stilbenes, lignans, lignins, suberins, cutins, flavonoids and tannins from phenylalanine. PAL denotes phenylalanine ammonia lyase.
BIOSYNTHESIS, CLASSIFICATION AND NOMENCLATURE
CINNAMIC AND BENZOIC ACID DERIVATIVES AND SIMPLE PHENOLS
Phenylalanine ammonia lyase (PAL) catalyzes the release of ammonia from phenylalanine and leads to the formation of a carbon-carbon double bond, yielding transcirmamic acid. In some plants and grasses tyrosine is converted into 4-hydroxycinnamic acid via the action of tyrosine ammonia lyase (TAL). Introduction of a hydroxyl group into the para position of the phenyl ring of cinnamic acid proceeds via catalysis by monooxygenase utilizing cytochrome P450 as the oxygen binding site. The p-coumaric acid formed may be hydroxylated further in positions 3 and 5 by hydroxylases and possibly methylated via O-methyl transferase with S-adenosylmethionine as methyl donor; this leads to the formation of caffeic, ferulic and sinapic acids (Figure 1.2 and Figure 1.3). These compounds possess a phenyl ring (C6) and a C3 side chain and are thus collectively termed phenylpropanoids, which serve as precursors for the synthesis of lignins and many other compounds.
Benzoic acid derivatives are produced via the loss of a two-carbon moiety from phenylpropanoids. Salicylic acid is a benzoic acid derivative that acts as a signal substance (Raskin, 1992). After infection or UV irradiation, many plants increase their salicylic acid content, which may induce the biosynthesis of defense substances. Aspirin, the acetyl ester of salicylic acid, was first isolated from the bark of the willow tree. Similar to phenylpropanoid series, hydroxylation and possibly methylation of hydroxybenzoic acid leads to the formation of dihydroxybenzoic acid (protocatechuic acid), vanillic acid, syringic acid and gallic acid (Figure 1.3). Hydroxybenzoic acids are commonly present in the bound form in foods and are often the component of a complex structure like lignins and hydrolyzable tannins. They are also found in the form of organic acids and as sugar derivatives (Schuster and Herrmann, 1985). However, exceptions in which they are present mainly in the free form do exist (Mosel and Herrmann, 1974a, b; Schmidtlein and Herrmann, 1975; Stöhr and Herrmann, 1975a, b).
Conventionally, phenylpropanoids (i.e., cinnamic acid family) and benzoic acid derivatives are collectively termed “phenolic acids” in the food science literature. However, it should be noted that this nomenclature is not necessarily correct from a chemical and structural viewpoint. Nonetheless, such compounds are referred to as phenolic acids in the remainder of this book.
Decarboxylation of benzoic acid and phenylpropanoid derivatives leads to the formation of simple phenols (Figure 1.2). Thermal degradation of lignin or microbial transformation may also produce simple phenols in foods (Maga, 1978); thus, vinylsubstituted phenols may be produced by decarboxylation of hydroxycinnamic acids (Pyysalo et al., 1977). However, exposure of 4-vinylguaiacol to oxygen leads to the formation of vanillin (Fiddler et al., 1967). A number of simple phenols, namely, phenol, o-cresol, 4-ethylphenol, guaiacol, 4-vinylguaiacol and eugenol, are found in foods of plant origin. Reduction products of phenylpropanoids also yie...
Table of contents
- COVER PAGE
- TITLE PAGE
- COPYRIGHT PAGE
- PREFACE
- AUTHORS
- 1. BIOSYNTHESIS, CLASSIFICATION, AND NOMENCLATURE OF PHENOLICS IN FOOD AND NUTRACEUTICALS
- 2. CEREALS, LEGUMES, AND NUTS
- 3. PHENOLIC COMPOUNDS OF MAJOR OILSEEDS AND PLANT OILS
- 4. PHENOLIC COMPOUNDS IN FRUITS AND VEGETABLES
- 5. PHENOLIC COMPOUNDS OF BEVERAGES
- 6. PHENOLICS IN HERBAL AND NUTRACEUTICAL PRODUCTS
- 7. NUTRITIONAL AND PHARMACOLOGICAL EFFECTS OF FOOD PHENOLICS
- 8. ANTIOXIDANT PROPERTIES OF FOOD PHENOLICS
- 9. CONTRIBUTION OF PHENOLIC COMPOUNDS TO FLAVOR AND COLOR CHARACTERISTICS OF FOODS
- 10 METHODS OF ANALYSIS AND QUANTIFICATION OF PHENOLIC COMPOUNDS