
- 456 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handbook of High-Temperature Superconductor
About this book
Devoted to the preparation, characterization and evaluation of HTS electronic devices, the Handbook of High-Temperature Superconductor Electronics provides information on using high-Tc thin films and junctions to increase speed, lessen noise, lower power consumption and enhance upper frequency limits in superconductor electronics. Compiled by a gro
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Handbook of High-Temperature Superconductor by Neeraj Khare in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Electrical Engineering & Telecommunications. We have over one million books available in our catalogue for you to explore.
Information
1
Introduction to High-Temperature Superconductors
Neeraj Khare
National Physical Laboratory, New Delhi, India
1.1 INTRODUCTION
The discovery of superconductivity in copper oxide perovskite (1) has opened a new era of research in superconducting materials. This class of materials not only show hightemperature superconductivity but also show properties that are different from classical superconductors. This offers a great challenge to understanding the basic phenomenon that causes superconductivity in these materials and to developing the appropriate preparation methods so that these can be exploited for a wide range of applications. During the last one and half decades after the discovery of high-Tc materials, several high-Tc superconductors have been discovered which show superconductivity at temperatures higher than liquid-nitrogen temperature (77K). There has also been great progress in understanding the properties of these materials, developing different methods of preparation, and realizing superconducting devices which use these superconductors.
This chapter will give a brief description of the historical developments in raising the transition temperature (Tc) of the superconductors, preparation, and structure of the material. Different properties of the high-Tc materials such as critical magnetic field, penetration depth, coherence length, critical current density, weak link, and so forth are also discussed.
1.2 RAISING THE TRANSITION TEMPERATURE
Superconductivity is the phenomenon in which a material loses its resistance on cooling below the transition temperature (Tc). Superconductivity was first discovered in mercury by Onnes (2) in 1911. The temperature at which mercury becomes superconducting was found to be close to the boiling point of liquid helium (4.2 K). Subsequently, many metals, alloys, and intermetallic compounds were found to exhibit superconductivity. The highest Tc known was limited to 23.2K (3) in the Nb3Ge alloy; however, in September 1986, Bednorz and Muller (1) discovered superconductivity at 30K in La-Ba-Cu-O. The phase responsible for superconductivity was identified to have nominal composition of La2−xBaxCuO4−y (x= 0.2). The discovery of high-temperature superconductivity in ceramic cuprate oxides by Bednorz and Muller led to unprecedented effort to explore new superconducting oxide material with higher transition temperatures. The value of Tc in La2−xBaxCuO4 was found to increase up to 57K with the application of pressure (4). This observation in La2−xBaxCuO4 material raised the hope of attaining even higher transition temperatures in cuprate oxides. This, indeed, turned out to be true when Chu and co-workers (5) reported a remarkably high superconductivity transition temperature (Tc) of 92K on replacing La by Y in nominal composition Y1.2Ba0.8CuO4−y. Later, different groups identified (6–8) that the superconducting phase responsible for 90K has the composition YBa2Cu3O7−y.
The discovery of superconductivity above the boiling point of liquid nitrogen led to extensive search for new superconducting materials. Superconductivity at transition temperatures of 105K in the multiphase sample of the Bi-Sr-Ca-Cu-O compound was reported by Maeda et al. (9) in 1988. The highest Tc of 110K was obtained in the Bi-Sr- Ca-Cu-O compound having a composition Bi2Sr2Ca2Cu3Oio (10, 11). Sheng and Hermann (12) substituted the nonmagnetic trivalent Tl for R in R-123, where R is a rareearth element. By reducing the reaction time to a few minutes for overcoming the highvolatility problem associated with T12O3, they detected superconductivity above 90K in TlBa2Cu3Ox samples in November 1987. By partially substituting Ca for Ba, they (13) discovered a Tc~120K in the multiphase sample of Tl-Ba-Ca-Cu-O in February 1988. In September 1992, Putillin et al. (14) found that the HgBa2CuOx (Hg-1201) compound with only one CuO2 layer showed a Tc of up to 94K. It was, therefore, rather natural to speculate that Tc can increase if more CuO2 layers are added in the per unit formula to the compound. In April 1993, Schilling et al. (15) reported the detection of superconductivity at temperatures up to 133K in HgBa2 Ca2Cu3Ox. The transition temperature of HgBa2Ca2Cu2Ox was found to increase to 153K with the application of pressure (16).
Figure 1.1 depicts the evolution in the transition temperature of superconductors starting from the discovery of superconductivity in mercury. The slow but steady progress to search for new superconductors with higher transition temperatures continued for decades until superconductivity at 30K in La-Ba-Cu-O oxide was discovered in 1986. Soon after this, other cuprate oxides such as Y-Ba-Cu-O, Bi-Sr-Ca- Cu-O, Tl-Ba-Ca-Cu-O with superconductivity above the liquid-nitrogen temperature were discovered.
Table 1.1 gives a list of some of high-Tc superconductors with their respective transition temperature, crystal structure, number of Cu-O layers present in unit cell, and lattice constants. Transition temperature has been found to increase as the number of Cu- O layer increases to three in Bi-Sr-Ca-Cu-O, Tl-Ba-Ca-Cu-O, and Hg-Ba-Ca-Cu-O compounds. In all of the cuprate superconductors described so far, the superconductivity is due to hole-charge carriers, except for Nd2−xCexCuO4 (Tc~20K), which is an n-type superconductor (17). The superconductor Ba0.6K0.4BiO3, which does not include Cu, was reported by Cava et al. (18) in 1988 exhibiting Tc~30K. A homologous series of compounds (Cu, Cr)Sr2Can−1CuxOy [Cr12(n−1)n] has been synthesized under high pressure.
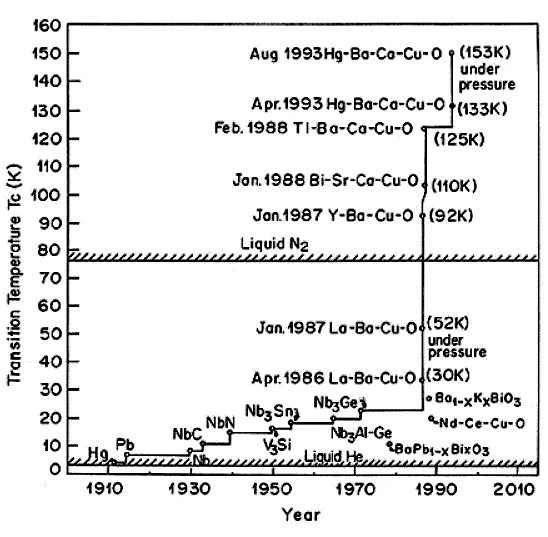
FIGURE 1.1 The evolution of the transition temperature (Tc) subsequent to the discovery of superconductivity.
TABLE 1.1 Transition Temperature (Tc), Crystal Structure and Lattice Constants of Some High-Tc Superconductors
In the Cr series, the value of n can be changed from 1 to 9, with a maximum Tc of 107K at n=3. The Pr(Ca)Ba2Cu3Oy compound has also been synthesized under high pressure, showing a transition temperature of 97K (19).
1.3 CRYSTAL STRUCTURE OF HIGH-Tc SUPERCONDUCTORS
The structure of a high-Tc superconductor is closely related to perovskite structure. The unit cell of perovskite consists of two metal (A, B) atoms and three oxygen atoms, with the general formula given as ABO3. The ideal perovskite structure is shown in Fig. 1.2a. Atom A, sitting at the body-centered site, is coordinated by 12 oxygen atoms. Atom B occupies the corner site and the oxygen atom occupies the edge-centered position. Figure 1.2b shows the unit cell of La2−xBaxCuO4, which has a tetragonal symmetry and consists of perovskite layers separated by rock-salt-like layers made of La (or Ba) and O atoms. This compound is often termed 214 because it has two La, one Cu, and four O atoms. The 214 compound has only one CuO2 plane. Looking at the exact center of Fig. 1.2b, the CuO2 plane appears as one copper atoms surrounded by four oxygen atoms, with one LaO plane above the CuO2 plane and one below it. The entire structure is layered. The LaO planes are said to be intercalated. The CuO2 plane is termed the conduction plane, which is responsible for superconductivity. The intercalated LaO planes are called “charge-reservoir layers.” When the intercalated plane contains mixed valence atoms, electrons are drawn away from the copper oxide planes, leaving holes to form pairs needed for superconductivity.
The structure of YBa2Cu3O7 is shown in Fig. 1.2c. The unit cell of YBa2Cu3O7 consists of three pseudocubic elementary perovskite unit cells (8). Each perovskite unit cell contains a Y or Ba atom at the center: Ba in the bottom unit cell, Y in the middle one, and Ba in the top unit cell. Thus, Y and Ba are stacked in the sequence [Ba-Y-Ba] along the c-axis. All corner sites of the unit eell are occupied by Cu, which has two different coordinations, Cu(1) and Cu(2), with respect to oxygen. There are four possible crystallographic sites for oxygen: O(1), O(2), O(3), and O(4). The coordination polyhedra of Y and Ba with respect to oxygen are different. The tripling of the perovskite unit cell (ABO3) leads to nine oxygen atoms, whereas YBa2Cu3O7 has seven oxygen atoms accommodating the deficiency of two oxygen atom...
Table of contents
- Cover Page
- Title Page
- Copyright Page
- Preface
- Contributors
- 1 Introduction to High-Temperature Superconductors
- 2 Epitaxial Growth of Superconducting Cuprate Thin Films
- 3 High-Temperature Superconducting Multilayer Ramp-Edge Junctions
- 4 Step-Edge Josephson Junctions
- 5 Conductance Noise in High-Temperature Superconductors
- 6 Noise in High-Temperature Superconductor Josephson Junctions
- 7 High-Temperature RF SQUIDs
- 8 High-Temperature SQUID Magnetometer
- 9 High-Temperature Superconducting Digital Circuits
- 10 High-Temperature Superconductor Microwave Devices
- 11 High-Temperature Superconducting IR Detectors
- 12 Cryocoolers and High-Tc Devices
- 13 High-Temperature Superconductor Electronics: Status and Perspectives