
- 760 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
The Medical Treatment of Epilepsy
About this book
This book is a comprehensive guide to the medical treatment of epilepsy and written for physicians in practice and those in training who take care of patients with seizures. It presents a review of every aspect of treatment that are considered prior to referring a patient for evaluation.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access The Medical Treatment of Epilepsy by Stanley R. Resor, Henn Kutt, Stanley R. Resor,Henn Kutt in PDF and/or ePUB format, as well as other popular books in Medicine & Nutrition, Dietics & Bariatrics. We have over one million books available in our catalogue for you to explore.
Information
V
Antiepileptic Drugs
27
Ethosuximide
Allan L. Sherwin
Montreal Neurological Institute and Hospital, Montreal, Quebec, Canada
I. Introduction
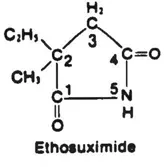
Ethosuxitnide (ESM) (see structure) is specifically indicated for the control of childhood absence epilepsy (petit mal, pyknolepsy), an age-related syndrome with onset between 4 and 8 years of age (1). Absence seizures are classified as a subgroup of the generalized epilepsies being bilaterally symmetrical without local onset. The unique EEG pattern differentiates absence seizures from other types of nonconvulsive attacks. Generalized spike-wave discharges reflect a widespread phase-locked oscillation between excitation (spike) and inhibition (wave) in mutually interconnected thalamo-cortical neuronal networks (2). The sudden blank stare suggests that absence seizures represent paroxysmal discharges in inhibitory pathways in the CNS. ESM acts by selectively depressing these low-frequency inhibitory pathways (3) possibly by selectively blocking the low threshold calcium channels of pacemaker neurons located in the intralaminar nucleus of the thalamus, which trigger and sustain rhythmic burst discharges (4).
II. Pharmacology
ESM is rapidly and completely absorbed from the gastrointestinal tract; clinically significant plasma concentrations are achieved within 1 hour following a single dose, whereas peak levels occur within 3 to 7 hours (5). The syrup is absorbed more rapidly than the capsules, but both formulations have equivalent total absorption, hence, patients can be switched from one form to the other, and dosage schedules remain unchanged. Steady-state plasma levels and urinary output are proportional to dose, being comparable when the drug is given as a single or divided dose. The apparent volume of distribution is 0.69 1/kg in children under 10 years of age and 0.62 1/kg in adults suggesting that the distribution of the drug conforms to that of total body water. ESM is not bound by plasma proteins and is distributed fairly uniformly, hence, the concentration in cerebrospinal fluid, saliva, and breast milk are similar to that of plasma. The drug is extensively metabolized by the liver so that less than 20% of the parent compound can be recovered from the urine. The elimination half-life of the drug in plasma following cessation of chronic therapy averages 30 hours in children, 41 hours in newborns and 60 hours in adults. The prolonged half-life and minimal diurnal variation indicates that once daily dosage is feasible. Steady state conditions are achieved after approximately 6 days in children and 12 days in adults. The therapeutic plasma level of ESM is considered to range from 40 to 100 μg/ml but in refractory cases levels of up to ISO p.g/ml may be required and are usually well tolerated (6,7).
III. Pregnancy
Placental transfer of both ESM and its metabolites has been reported, and there is a risk of congenital malformations in infants exposed to the drug in utero. We monitored plasma drug levels of five patients receiving polytherapy that included ESM during pregnancy, one of whom had a malformed child. Her mean dosage (17.3 mg/kg) and mean plasma level (45.2 μg/ml) were about twice the corresponding mean values for the mothers of normal children (8). Because absence seizures tend to remit at puberty or become relatively mild it is usually possible to discontinue ESM therapy preferably one month before conception. If drug therapy must be continued during pregnancy plasma ESM levels should be kept as low as possible. Breast milk concentrations of ESM are similar to those in maternal plasma and nursed infants were reported to maintain plasma levels between 15 and 40 μg/ml (9).
IV. Clinical Use
ESM has proved to be effective in absence seizures of both the typical and atypical types (Table 1). It can be employed as monotherapy in the majority of children presenting with absence attacks without a history of generalized tonic-clonic seizures (GTCSs), though the parents should be informed that GTCSs occur at some time in over one third of the patients. It can readily be combined with carbamazepine or phenytoin (PHT) because it does not induce hepatic drug-metabolizing enzymes and rarely causes clinically significant drug interactions. If GTCSs have already occurred, valproate (VPA) monotherapy that is efficacious against both seizure types is recommended. There are no significant differences in efficacy between ESM and VPA in groups of patients with simple absence seizures (7). In clinical practice, however, individual patients may respond better to one or the other agent in monotherapy. ESM does not cause excessive weight gain, which may be a consideration in obese patients. Patients with refractory absence seizures may respond favorably to ESM and VPA in combination. This is particularly the case in atypical absence seizures and myoclonic absences (10,11). VPA decreases the total body clearance of ESM, which can result in a significant rise in ESM plasma levels, which should be monitored to avoid drug toxicity (12). Thus a lower dosage of ESM is frequently efficacious in combination with VPA and should be prescribed if monotherapy with either agent has failed to fully control the attacks.
|
ESM is available as a 250 mg capsule or as a flavored syrup. Because the formulations have equal bioavailability, there is no difficulty in switching from one to the other. Therapy can be instituted by starting with one capsule after supper and increasing the dosage every 3 days until a maintainence dosage of 15 to 20 mg/kg/day is reached. The drug can be given once daily but many patients prefer to take the capsules in two or three divided doses after meals. Steady state will be achieved about one week after full dosage is administered. Because of the long-life ESM levels fluctuate little from day to day and the timing of plasma level monitoring is not critical. It is advisable to maintain a dosage sufficient to achieve a plasma level greater than 40 μg/ml for approximately a month. The parents should be asked to record the frequency of attacks; then the dosage can be increased at monthly intervals up to 40 mg/kg in children. ESM is metabolized at a rather constant rate, and plasma concentrations are relatively unaffected by the presence of other AEDs. Steady-state therapeutic levels, in the main, depend on regular intake of an adequate dosage based on body weight. There is a significant relationship between ESM dosage in mg/kg and body weight. The administration of ESM at 20 mg/kg in boys or girls under 11 years of age will result in mean plasma concentrations of approximately 50 μg/ml. It is very important to anticipate the increased dosage requirement due to growth or weight gain so as to maintain effective plasma concentrations. Once attained, it would be unfortunate to lose good seizure control. Plasma ESM levels should be monitored monthly until seizure control is attained, then at 3-month intervals. Noncompliance is a particular problem here because absence attacks are much less frightening to both parents and teachers than are GTCSs.
V. Dose-Related Adverse Effects
Approximately 20% of patients will experience dose-related adverse effects. These include gastric distress, nausea, vomiting, drowsiness, and anorexia. There is also a somewhat vague syndrome that includes fatigue, lethargy, dizziness, and even euphoria. These symptoms usually subside with time, although it may be necessary to reduce the dosage considerably a period of 1 to 2 weeks. The dosage can then be gradually augmented until seizure control is attained. Headaches, often occipital in location, can occur, and hiccups are also occasionally reported. As they do not always respond to lowered dosage it is uncertain whether headaches should be grouped with the dose related or idiosyncratic adverse effects (11).
VI. Non-Dose Related Adverse Effects
Non-dose related adverse effects forcing discontinuation of therapy include skin rashes mostly nonspecific in nature but erythema multiforme, the Stevens-Johnson syndrome, and scleroderma (13). Hematologic prob...
Table of contents
- Cover
- Half Title
- Series Page
- Title Page
- Copyright Page
- Introduction to the Series
- Foreword
- Preface
- Table of Contents
- Contributors
- Original Half Title
- I. General Principles
- II. Treatment Overview
- III. Treatment of Nonepileptic Events Presenting as Seizures
- IV. When to Consider a Surgical Evaluation
- V. Antiepileptic Drugs
- VI. Other Treatments
- VII. Drugs for Myoclonus
- VIII. Special Problems
- IX. Investigational Treatments
- X. Legal and Regulatory Issues
- Index
- About the Editors