
- 718 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Fundamental Physics of Radiology
About this book
Fundamental Physics of Radiology, Third Edition provides a general introduction to the methods involving radioactive isotopes and ultrasonic radiations. This book provides the fundamental principles upon which the clinical uses of radioactive isotopes and ultrasonic radiation depend. Organized into four sections encompassing 45 chapters, this edition begins with an overview of the basic facts about matter and energy. This text then examines the technical details of some practical X-ray tubes. Other chapters consider the action of the X-rays on the screen to produce an emission of visible light photons in amount proportional to the incident X-ray intensity. This book discusses as well the fundamental aspects of the physical principles of radiotherapy, in which most attention is being given to gamma- and X-rays. The final chapter deals with the provision of adequate barriers and protective devices to guarantee the safety of the workers concerned. This book is a valuable resource for radiologists, physicists, and scientists.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
MATTER AND ENERGY, RADIATION AND SPECTRA
Publisher Summary
ENERGY
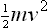
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface to The Third Edition
- Preface to The First Edition
- CHAPTER I : MATTER AND ENERGY, RADIATION AND SPECTRA
- CHAPTER II : ATOMS AND NUCLEI
- CHAPTER III : RADIOACTIVITY
- CHAPTER IV : RADIOACTIVITY—MATERIALS
- CHAPTER V : THE PRODUCTION OF X-RAYS
- CHAPTER VI : THE INTERACTION OF X- AND GAMMA RAYS WITH MATTER—I
- CHAPTER VII : THE INTERACTION OF X- AND GAMMA RAYS WITH MATTER—II
- CHAPTER VIII : THE EFFECTS OF X-RAYS
- CHAPTER IX : THE MEASUREMENT OF X-RAY QUANTITY
- CHAPTER X : THE ROENTGEN AND ITS MEASUREMENT
- CHAPTER XI : THE GEIGER-MÜLLER AND SCINTILLATION COUNTERS AND THE THERMOLUMINESCENCE DOSEMETER
- CHAPTER XII : ABSORBED DOSE AND THE RAD
- CHAPTER XIII : FILTERS AND FILTRATION
- CHAPTER XIV : THE PHYSICAL BASIS OF DIAGNOSTIC RADIOLOGY
- CHAPTER XV : THE X-RAY FILM AND ITS PROCESSING
- CHAPTER XVI : THE PROPERTIES OF THE X-RAY FILM
- CHAPTER XVII : INTENSIFYING AND FLUORESCENT SCREENS AND XERORADIOGRAPHY
- CHAPTER XVIII : GEOMETRIC FACTORS WHICH INFLUENCE THE RADIOGRAPHIC IMAGE
- CHAPTER XIX : THE EFFECT OF X-RAY ABSORPTION ON THE RADIOGRAPHIC IMAGE
- CHAPTER XX : THE EFFECTS AND CONTROL OF SCATTERED RADIATION
- CHAPTER XXI : THE RADIOGRAPHIC EXPOSURE
- CHAPTER XXII : THE DIAGNOSTIC X-RAY TUBE AND SHIELD
- CHAPTER XXIII : THE ELECTRICAL CIRCUITS OF THE X-RAY UNIT
- CHAPTER XXIV : THE RATING OF THE X-RAY TUBE
- CHAPTER XXV : FLUOROSCOPY
- CHAPTER XXVI : TOMOGRAPHY
- CHAPTER XXVII : ULTRASONICS IN CLINICAL MEDICINE
- CHAPTER XXVIII : RADIOACTIVE ISOTOPES IN CLINICAL MEDICINE
- CHAPTER XXIX : THE PHYSICAL PRINCIPLES OF RADIOTHERAPY
- CHAPTER XXX : TELETHERAPY DOSAGE DATA: GENERAL CONSIDERATIONS
- CHAPTER XXXI : TELETHERAPY DOSAGE DATA FOR CLINICAL USE
- CHAPTER XXXII : OUTPUT MEASUREMENTS AND THE USE OF ISODOSE CHARTS
- CHAPTER XXXIII : PATIENT DOSAGE
- CHAPTER XXXIV : BEAM MODIFICATION
- CHAPTER XXXV : COLLIMATORS AND ‘BEAM-DIRECTION’ DEVICES
- CHAPTER XXXVI : THE TREATMENT PRESCRIPTION
- CHAPTER XXXVII : SOME SPECIAL TECHNIQUES
- CHAPTER XXXVIII : TELETHERAPY SOURCES
- CHAPTER XXXIX : ACCEPTANCE TESTS AND CALIBRATION
- CHAPTER XL : GAMMA-RAY SOURCES FOR PLESIOTHERAPY
- CHAPTER XLI : PLESIOTHERAPY DOSAGE CALCULATIONS
- CHAPTER XLII : PARTICLE RADIATIONS IN RADIOTHERAPY
- CHAPTER XLIII : GENERAL PRINCIPLES AND MATERIALS
- CHAPTER XLIV : DEPARTMENTAL PROTECTION
- CHAPTER XLV : PROTECTION INSTRUMENTS AND PERSONNEL MONITORING
- APPENDIX I: S.I. UNITS
- APPENDIX II: THE EXPONENTIAL LAW OF RADIOACTIVE DECAY, OR OF PHOTON BEAM ATTENUATION
- APPENDIX III: MODULATION TRANSFER FUNCTION AND ASSOCIATED CONCEPTS
- INDEX