
- 746 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Introduction to Medical Laboratory Technology
About this book
Introduction to Medical Laboratory Technology presents the development in the medical laboratory science. It discusses the general laboratory glassware and apparatus. It addresses a more specialized procedure in mechanization, automation, and data processing. Some of the topics covered in the book are the composition of glass; cleaning of glassware; the technique of using volumetric pipettes; technique for centrifugation; the production of chemically pure water; principal foci of a converging lens; micrometry; magnification; setting up the microscope; and fluorescence microscopy. The precautions against infection are covered. The storage of chemicals and treatment of accidents are discussed. The text describes the collection and reporting of specimens. A study of the fundamentals of chemistry and endocrine systems is presented. A chapter is devoted to the elementary colorimetry and spectro-photometry. Another section focuses on the introduction to clinical chemistry and blood gas analysis. The book can provide useful information to scientists, physicists, doctors, students, and researchers.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Information
Some Fundamentals of Chemistry
Publisher Summary
1 Law of Conservation of Mass
2 Law of Constant Composition
3 Law of Multiple Proportions
4 Law of Reciprocal Proportions (or equivalent proportions)
Equivalents
THE GAS LAWS
1 Boyle’s Law
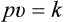
Table of contents
- Cover image
- Title page
- Table of Contents
- Inside Front Cover
- Copyright
- Preface
- Introduction
- SECTION ONE: GENERAL
- SECTION TWO: CLINICAL CHEMISTRY
- SECTION THREE: HISTOLOGY
- SECTION FOUR: MICROBIOLOGY
- SECTION FIVE: HAEMATOLOGY
- SECTION SIX: BLOOD TRANSFUSION TECHNIQUE
- Bibliography
- Appendix
- Glossary
- Index