
- 368 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Modern Batteries
About this book
Based on the successful first edition, this book gives a general theoretical introduction to electrochemical power cells (excluding fuel cells) followed by a comprehensive treatment of the principle battery types - covering chemistry, fabrication characteristics and applications. There have been many changes in the field over the last decade and many new systems have been commercialised. Since the recent advent of battery powered consumer products (mobile phones, camcorders, lap-tops etc.) advanced power sources have become far more important. This text provides an up-to-date account of batteries which is accessible to anyone with a basic knowledge of chemistry and physics.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Introduction
Colin A Vincent
1.1 Electrochemical power sources
An electrochemical power source or battery is a device which enables the energy liberated in a chemical reaction to be converted directly into electricity. Batteries fulfil two main functions. First and foremost they act as portable sources of electric power. Well known modern examples range from the small button cells used in electric watches to the lead–acid batteries used for starting, lighting and ignition in vehicles with internal combustion engines. The second function, which is likely to increase in importance over the next 20 years, is based on the ability of certain electrochemical systems to store electrical energy supplied by an external source. Such batteries may be used for driving electric vehicles, for emergency power supplies, and as part of the main electricity supply system for meeting short duration demand peaks (load levelling) or in conjunction with renewable energy sources, such as solar, wave or wind power.
The first authenticated description of an electrochemical battery was given by Alessandro Volta, Professor of Natural Philosophy (Physics) at the University of Pavia in Italy, in a letter to the Royal Society (London) in 1800. A photograph of an original Volta ‘pile’ is shown in Fig. 1.1. The importance of Volta’s discovery as a tool for advancing the understanding of chemistry and physics was immediately grasped by scientists in a number of countries. However, it was the introduction of telegraph systems, which were becoming of increasing importance in the 1830s, that gave rise to the development of reliable commercial batteries, capable of sustaining a substantial flow of current without undue loss of cell voltage.
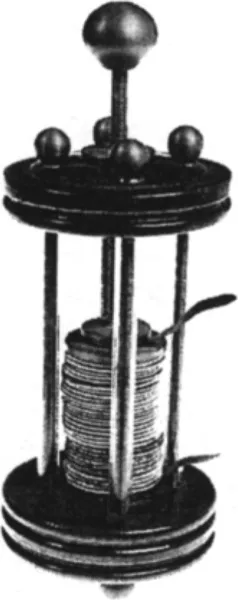
Fig. 1.1 Volta pile
The first high current electroplating battery was described in 1840, and over the next two decades the use of techniques such as electroplating and electroforming, together with the exploitation of practical electric motors, gradually became more widespread. In the 1870s, a more general consumer market for batteries was created by the manufacture of electric bell circuits for homes, offices and hotels. The ‘flashlight’ was introduced at the turn of the century, some 20 years after Edison’s invention of the incandescent lamp. By then the annual production of batteries in the USA alone had exceeded two million units.
The large-scale introduction from 1870 onwards of dynamos or electromagnetic generators driven by heat engines led to the worldwide industrial and domestic use of ‘mains’ electricity, accepted as commonplace today, a century later. This ready availability of electrical energy was the principal motivation for the development of secondary or storage batteries, although this area was soon to be further stimulated by the demands of the growing motor car industry.
A further impetus for commercial battery development came with the introduction of domestic radio receivers in the 1920s, and an equivalent growth has been seen over the last 30 years with the development of microelectronics-based equipment. Today, it is estimated that annual battery production totals 8–15 units per head of population throughout the developed countries of the world.
The world market for batteries of all types now exceeds £100 billion. Over half of this sum is accounted for by lead–acid batteries – mainly for vehicle starting, lighting and ignition (SLI), and industrial use including traction and standby power, with about one-third being devoted to primary cells and the remainder to alkaline rechargeable and specialist batteries.
1.2 Nomenclature
There is some confusion in the terminology used to denote the electrochemical devices which convert chemical into electrical energy. In many cases, the devices have changed in character with the passage of time, but have retained their original names; in others, the terms commonly used do not clearly define the nature of the device. In this book we conform to general usage and employ the words ‘cell’ and ‘battery’ interchangeably to describe a closed electrochemical power source, i.e. one in which the reactants are incorporated during manufacture. The term ‘battery’ originally implied a group of ‘cells’ in a series or parallel arrangement, but is now understood to mean either a single cell or a group of cells.
Two other terms which are not self-explanatory are ‘primary’ and ‘secondary’ cells. A primary system is one whose useful life is ended once its reactants have been consumed by the discharge process. In contrast, a secondary system is capable of being charged or recharged when its reactants have been used up: the spontaneous electrochemical reaction can be reversed by passing current through the cell in the opposite direction to that of cell discharge. A secondary battery might therefore be considered as an electrochemical energy storage unit. Note, however, that the energy derived from the external current is stored as chemical energy, and not as electrical energy as in a capacitor. Other terms are sometimes used to describe this system, e.g. accumulator (introduced by Davy along with the terms ‘cell’ and ‘circuit’), storage battery, rechargeable battery, etc. In a reserve cell, one component (usually the electrolyte) is separated from the rest of the battery or maintained in an inactive condition until the cell is activated. Such cells are capable of very long-term storage in environmentally hostile conditions since self-discharge and other chemical processes are minimized. Reserve cells are used in applications such as life-jacket or life-raft lights, or in missile weapon systems.
We do not consider the related subject of fuel cells, where both cathodic and anodic reagents – usually gases – are stored externally and can be supplied to the electrochemical cell on a continuous basis. A number of books have recently been published on this topic. The term ‘hybrid cell’ is used here to describe a power source in which one of the active reagents is in the gaseous state, e.g. the oxygen of the air. Use of the word ‘hybrid’ in this context should not be confused with its meaning in the phrase ‘hybrid electric vehicle’, which refers to an electric vehicle with more than one power supply, as described below.
A large number of technical terms are associated with the literature on batteries: the more common of these are given in the Glossary, while the electrical quantities used to describe battery performance and characteristics are defined in Section 2.5, and summarized in Appendix 4.
The most common convention for writing an electrochemical cell is to place the negative electrode on the left and the positive on the right. The cell is then named in the same way: thus reference to the ‘sodium–sulphur cell’ implies that sodium is the active reagent at the negative electrode and sulphur is that at the positive electrode. We make three exceptions to this general rule in order to conform to normal usage, and call the lead–lead oxide cell the ‘lead–acid cell’, the cadmium–nickel oxide cell the ‘nickel–cadmium cell’, and the zinc–manganese dioxide cell the ‘Leclanché cell’.
1.3 The renaissance in battery development
Until very recently, ‘conventional’ batteries using solid electrodes and aqueous electrolytes proved satisfactory for the majority of common applications. Traditional primary systems, such as the Leclanché Zn-MnO2 cell and the alkaline manganese cell, have been (and to a large extent still are) adequate power sources for portable electrical equipment. Well established rechargeable batteries such as those based on the lead–acid or nickel–cadmium systems have for a long time been employed as small localized energy storage units (e.g. in rural areas, in railway and telephone systems, etc.) and as sources of auxiliary power in ground, air and sea transport. For many years, research and development in the battery industry has been directed mainly towards improvements in these well known systems, especially in the fields of engineering design and production.
In the past 25 years, however, the situation has changed considerably. First, advances in semiconductor technology have led to the production of large-scale integrated (LSI, VLSI and ULSI) circuits in immense numbers, bringing about a revolution in the electronics industry. Microelectronic components are now inexpensive and are widely used in the production of pocket calculators, electric watches and similar devices. In 1990, the world production of battery-powered watches was 4 × 108. Development of a wide variety of such electronics-based consumer products soon demanded the evolution of miniature power supplies which would offer a much higher energy per unit volume and superior discharge characteristics as compared with those of traditional batteries.
The second, and perhaps more important, factor affecting the demand for new battery systems was the realization in the late 1960s that the constantly increasing energy needs of the developed countries of the world would soon lead to the progressive exhaustion of oil supplies. This in turn led to the requirement for more efficient use of the remaining fossil fuel reserves and for a shift towards the exploitation of alternative energy sources, preferably of a clean and regenerative type. Central to the problem both of the utilization of discontinuous energy sources, e.g. solar, wind and wave power, and of the efficient running of conventional generating plant is the provision of suitable energy storage systems. While there are a number of alternatives to be considered, such as pumped hydroelectric or compressed air storage, electrochemical storage batteries are in many instances more convenient, being transportable and flexible in size, as well as being silent and non-polluting. For this application, batteries require the ability to undergo large numbers of deep charge/discharge cycles with high efficiency and without physical degradation.
Since a considerable proportion of all petroleum is consumed in vehicle traction – a particularly inefficient way of extracting energy from a scarce resource which simultaneously causes severe environmental pollution in urban areas – the possibility of replacing vehicles driven by internal combustion engines with battery-powered electric transport is under active consideration, and the development of advanced batteries for this purpose is being pursued in a number of countries. Since batteries for electric vehicles (EVs) must be transported as part of the vehicle load, they require high power/mass ratios in addition to high cycle efficiency.
1.4 A survey of common battery types and applications
The total available energy of a battery is a measure of how much electricity it can deliver (usually measured in Wh) and is directly related to the size of the unit. One possible classification of the most common commercial battery types, according to size, is given in Table 1.1. The range of battery energies extends over at least 15 orders of magnitude. At the bottom of the range there are 0.1 cm2 experimental cells with a PbF2 solid electrolyte which have a total energy of just over 1 × 10− 6 Wh. The smallest commercial button cells have energies of around 100 mWh, while the common ‘D size’ cylindrical cells, which have a total volume of 45 cm3, have energies in the range of 2–15 Wh. Rechargeable cells used in power tools and other ‘cordless’ electric appliances can supply 20–100 Wh. At the top of the range, submarine lead–acid batteries weighing nearly 200 tonnes have rated energies of 3 MWh, while the load levelling batteries of 40 MWh have now been realized.
Table 1.1
Classification of batteries according to size
| Type | Energy | Applications |
| Miniature batteries | 100 mWh–2 Wh | Electric watches, calculators, implanted medical devices |
| Batteries for portable equipment | 2 Wh–100 Wh | Flashlights, toys, power tools, portable radio and television, mobile phones, camcorders, lap-top computers |
| SLI batteries (starting, lighting and ignition) | 100–600 Wh | Cars, trucks, buses, tractors, lawn mower traction |
| Vehicle traction batteries | 20–630 kWh (3 MWh) | Fork-lift trucks; milk floats, locomotives (submarines) |
| Station... |
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright page
- Preface to the second edition
- Preface to the first edition
- 1: Introduction
- 2: Theoretical background
- 3: Primary aqueous electrolyte cells
- 4: Primary lithium cells
- 5: Secondary lead-acid cells
- 6: Secondary alkaline cells
- 7: Rechargeable lithium cells
- 8: High temperature cells
- 9: Miscellaneous cells
- Appendix 1: Operational modes and charging techniques for secondary batteries
- Appendix 2: Nomenclature and standardization systems for small cells and batteries
- Appendix 3: Waste disposal and the recycling of batteries
- Appendix 4: Electrical quantities, physical constants and conversion factors
- Bibliography
- Glossary
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Modern Batteries by C. Vincent,Bruno Scrosati in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Electrical Engineering & Telecommunications. We have over one million books available in our catalogue for you to explore.