
- 244 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Ultrafiltration for Bioprocessing
About this book
Ultrafiltration for Bioprocessing is key reading for all those involved in the biotechnology and biopharmaceutical areas. Written by a leading worker in the area, it includes many practical applications and case studies in the key process of ultrafiltration (UF), which is used in almost every bioprocess.
- Focuses on ultrafiltration for biopharmaceuticals—other books look at general ultrafiltration or general biopharmaceuticals
- A mix of theory and practical applications—other books tend to be more theory-oriented
- Addresses the main issues encountered in development and scale-up through recommendations and case studies
Information
1
Fundamentals
Herb Lutz EMD Millipore, Biomanufacturing Sciences Network
Abstract
This chapter provides a high-level overview of ultrafiltration. This includes basic terminology, principles of operation, performance characteristics, and applications where it is used. It is intended to provide an overall perspective and furnish a background to enable the reader to more readily understand the subsequent in-depth chapters.
Keywords
cross-flow filtration
filtrate
flux
formulation
NFF
permeate
retentate
tangential flow filtration
TFF
TMP

ultrafiltration
UF applications
UF membrane
UF operation
UF processing steps
UF system
1.1. Membrane pore sizes
A membrane can be idealized as a film that readily passes solvents and small solutes but retains large solutes above a particular size. UF (Ultrafiltration) membranes retain solutes with hydraulic diameters in the 5–150 nm range. This roughly corresponds to molecular weights in the 1–1000 kDa range covering most proteins, nucleic acids, nanoparticles, viruses, and some polymers. In the field of membranes, the term nanofiltration refers to membranes tighter than ultrafiltration but more open than reverse osmosis (Figure 1.1). However, in biotechnology, it has come to refer to virus-retaining membranes that fall in the open ultrafiltration (roughly 100 kDa) to tight microfiltration range (roughly 0.1 μm).
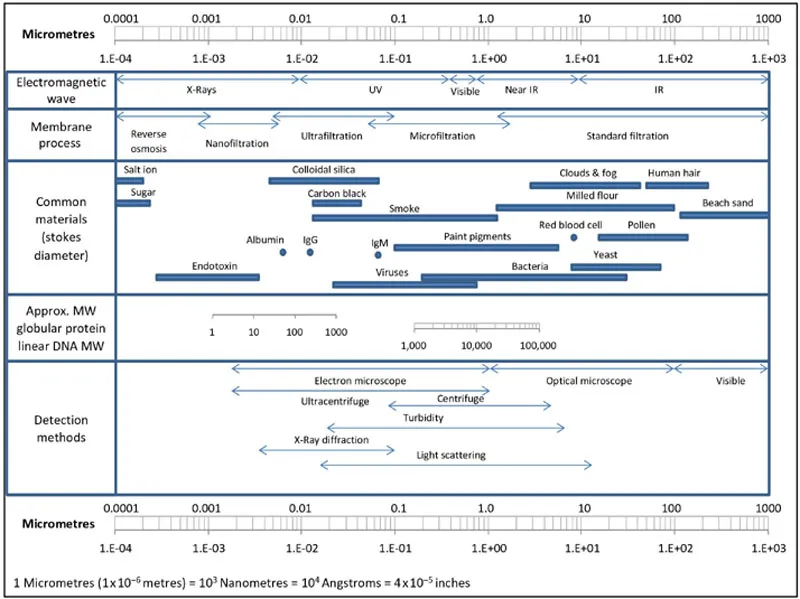
Figure 1.1 Ultrafiltration size range.
1.2. Applications
In a biopharmaceutical manufacturing process, biopharmaceutical products are expressed in a bioreactor (for mammalian cells) or fermentor (for bacterial or yeast cells). Ultrafiltration has been used to retain colloids and cell debris while passing the expressed product. In a similar way, ultrafiltration has been used after a refold pool to retain refold aggregates while passing the refolded product. Following clarification, ultrafiltration has been used to remove the largest contaminant – water – by retaining the product in a concentration step. This can be to reduce the size of subsequent steps, which may have to be sized based on the batch volume rather than on product mass. In addition, volume reduction facilitates transport and storage. This can provide flexibility to a manufacturing operation by decoupling the upstream from downstream operations.
Ultrafiltration is used to purify large solutes, such as vaccines, by retaining the desired product and passing through unwanted smaller components. This can include passing unreacted PEG or unconjugated polymers. Ultrafiltration is also used to retain unwanted viruses or aggregates while letting the desired product pass through. When the desired product and unwanted solutes are close in size, this is a challenging fractionation operation. This book will not cover virus ultrafiltration, or nanofiltration as it is frequently called.
The most common application is the use of ultrafiltration for final product formulation. This involves retaining the product while concentrating it to the desired target, and conducting a buffer exchange using a diafiltration process. Small contaminants and the old buffer components pass through the membrane.
1.3. Modes of operation
Membranes are encased in modules for ease-of-operation. One could run the membrane in a static or dialysis mode where small buffer solutes can be exchanged by diffusion across the membrane (Figure 1.2). This is convenient at the bench scale but economical commercial operation requires bulk flow. UF can also be run in NFF (normal flow filtration) or TFF (tangential flow filtration) modes. NFF (Figure 1.2a) is the most common type of filtration. NFF mode involves passing the solvent through the filter under pressure where the fluid velocity is perpendicular (or normal) to the plane of the membrane. As the fluid passes through the membrane, it drags solutes with it to the membrane surface where they accumulate and cause the filter hydraulic resistance to increase. For high concentrations of retained solutes, NFF operation leads to relatively rapid plugging and low filter capacities. NFF is used at bench scale where low capacities pose less of a concern and the ease-of-use is convenient. NFF is also used for virus filtration.
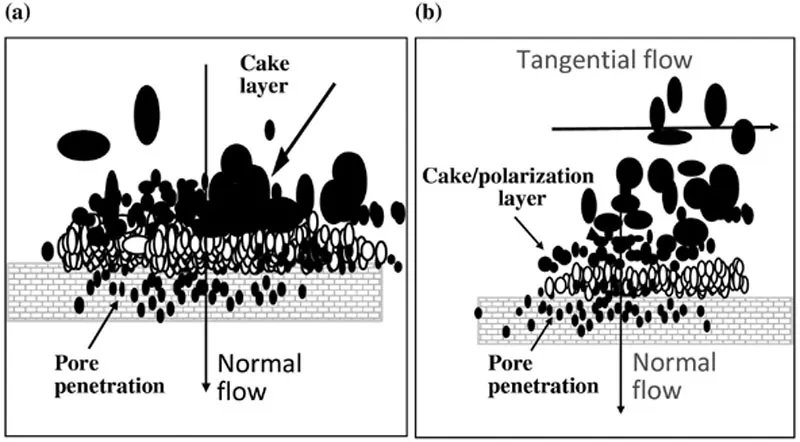
Figure 1.2 Operating modes.
TFF mode (Figure 1.2b) involves adding another fluid velocity component parallel to the plane of the membrane so the net solvent flow strikes the membrane at an angle. The presence of the tangential flow at the membrane surface facilitates backflow of solutes and prevents filter plugging. While there remains a region of high solute concentration at the membrane surface, steady-state operation is reached and TFF operation shows very high filter cap...
Table of contents
- Cover
- Title page
- Table of Contents
- Copyright
- About the contributors
- About the editor
- Preface
- Acknowledgments
- 1: Fundamentals
- 2: Membranes
- 3: Modules
- 4: Module performance
- 5: Configurations
- 6: Pre-processing operations
- 7: Processing
- 8: Post-processing
- 9: Systems
- 10: Validation
- 11: Troubleshooting
- 12: Conclusion
- Appendix A
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Ultrafiltration for Bioprocessing by Herb Lutz,H Lutz,Herbert Lutz in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Biotechnology. We have over one million books available in our catalogue for you to explore.