
- 312 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Corrosion of Steel in Concrete Structures
About this book
Corrosion of reinforcing steel is now recognized as the major cause of degradation of concrete structures in many parts of the world. Despite this, infrastructure expenditure is being unreasonably decreased by sequestration and the incredible shrinking discretionary budget. All components of our infrastructure including highways, airports, water supply, waste treatment, energy supply, and power generation require significant investment and are subjected to degradation by corrosion, which significantly reduces the service life, reliability, functionality of structures and equipment, and safety. Corrosion of Steel in Concrete Structures provides a comprehensive review of the subject, in addition to recent advances in research and technological developments, from reinforcing materials to measurement techniques and modelling.This book contains not only all the important aspects in the field of corrosion of steel reinforced concrete but also discusses new topics and future trends. Part One of the book tackles theoretical concepts of corrosion of steel in concrete structures. The second part moves on to analyse the variety of reinforcing materials and concrete, including stainless steel and galvanized steel. Part Three covers measurements and evaluations, such as electrochemical techniques and acoustic emission. Part Four reviews protection and maintenance methods, whilst the final section analyses modelling, latest developments and future trends in the field.The book is essential reading for researchers, practitioners and engineers who are involved in materials characterisation and corrosion of steel in concrete structures.- Provides comprehensive coverage on a broad range of topics related to the corrosion of steel bars in concrete- Discusses the latest measuring methods and advanced modeling techniques- Reviews the range of reinforcing materials and types of concrete
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
An introduction to corrosion of engineering materials
Abstract
Keywords
Cost of corrosion; Electrolytic (stray current) corrosion; General corrosion; Pitting; Pourbaix diagrams1.1. Introduction—the ubiquitous nature of corrosion
1.2. Thermodynamics are on the side of corrosion
| Electrode type | Equilibrium reactions Nernst equation | Conditions (activity) | Potential V vs SHE | Temp. coefficient mV/°C |
| Standard hydrogen electrode (SHE) | 2H+ + 2e− = H2 | pH = 0 | ||
| E0 = 0.059 pH | ||||
| Silver chloride | AgCl + e− = Ag + Cl− | aCl− = 1 | 0.2224 | −0.6 |
| E0 = 0.059log10(aCl−) | 0.1 M KCl | 0.2881 | −0.6 | |
| 1.0 M KCl | 0.235 | −0.6 | ||
| Saturated KCl | 0.199 | −0.6 | ||
| Seawater | ∼0.250 | −0.6 | ||
| Calomel | Hg2Cl2 + 2e− = 2Hg + 2Cl− | aCl− = 1 | 0.268 | |
| E0 − 0.059log10(aCl−) | 0.1 M KCl | 0.3337 | −0.06 | |
| 1.0 M KCl | 0.280 | −0.24 | ||
| Saturated KCl | 0.241 | −0.65 | ||
| Mercurous sulfate | Hg2SO4 + 2e− = 2Hg + SO42− | 0.6151 | ||
| E0 − 0.0295log10(aSO42−) | ||||
| Mercuric oxide | HgO + 2e− + 2H+ = Hg + H2O | 0.926 | ||
| E0 − 0.059 pH | ||||
| Copper sulfate | Cu2+ + 2e− = Cu (SO2 solution | aCu = 1 | 0.340 | |
| E0 + 0.0295log10(aCu2+) | Saturated | 0.318 |
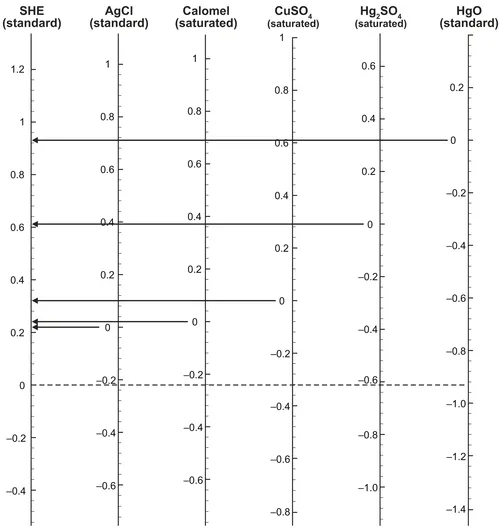
Table of contents
- Cover image
- Title page
- Table of Contents
- Related titles
- Copyright
- List of contributors
- Woodhead Publishing Series in Civil and Structural Engineering
- Preface
- Part One. Theoretical concepts of corrosion of steelin concrete structures
- Part Two. Different reinforcing materials and concrete
- Part Three. Measurements and evaluations
- Part Four. Protection, modeling and future trends
- Index
