
- 794 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This is the first book that can be considered a textbook on thin film science, complete with exercises at the end of each chapter. Ohring has contributed many highly regarded reference books to the AP list, including Reliability and Failure of Electronic Materials and the Engineering Science of Thin Films. The knowledge base is intended for science and engineering students in advanced undergraduate or first-year graduate level courses on thin films and scientists and engineers who are entering or require an overview of the field.
Since 1992, when the book was first published, the field of thin films has expanded tremendously, especially with regard to technological applications. The second edition will bring the book up-to-date with regard to these advances. Most chapters have been greatly updated, and several new chapters have been added.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Materials Science of Thin Films by Milton Ohring in PDF and/or ePUB format, as well as other popular books in Tecnologia e ingegneria & Chimica fisica e teoretica. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1 A Review of Materials Science
1.1 INTRODUCTION
A cursory examination of the vast body of solid substances reveals what outwardly appears to be an endless multitude of external forms and structures possessing a bewildering variety of properties. The branch of study known as Materials Science evolved in part to classify those features that are common among the structure and properties of different materials in a manner somewhat reminiscent of chemical or biological classification schemes. This dramatically reduces the apparent variety. From this perspective, it turns out that solids can be classified as belonging typically to one of only four different categories (metallic, ionic, covalent, van der Waals) depending on the nature of the electronic structure and resulting interatomic bonding forces. Another scheme based on engineering use would again arguably limit materials to four chief classes, namely, metals, semiconductors, polymers, and ceramics.
Similar divisions occur with respect to structure of solids. Solids are either internally crystalline or noncrystalline. Those that are crystalline can be further subdivided according to one of 14 different geometric arrays or lattices depending on the placement of the atoms. When properties are considered, there are similar descriptors and simplifying categorizations. Thus, materials are either good, intermediate, or poor conductors of electricity, they are either mechanically brittle or can easily be stretched without fracture, they are either optically reflective or transparent, etc. It is, of course, easier to recognize that property differences exist than to understand why they exist. Nevertheless, much progress has been made in this subject as a result of the research of the past century. Basically, the richness in the diversity of materials properties occurs because countless combinations of the admixture of chemical compositions, bonding types, crystal structures, and morphologies either occur naturally or can be synthesized.
This chapter reviews various aspects of the structure, bonding, and properties of solids with the purpose of providing the background to better understand the remainder of the book. Additional topics dealing with thermodynamics and kinetics of atomic motion in materials are also included. These will later have relevance to aspects of the formation, stability, and solid-state reactions in thin films. This review ends with a discussion of mechanical properties, a subject of significance in phenomena ranging from film deposition to adhesion. Although much of this chapter is a condensed adaptation of standard treatments of bulk materials, it is largely applicable to thin films as well. Nevertheless, many distinctions between bulk materials and films exist and they will be stressed in the ensuing discussion. Readers already familiar with concepts of materials science may wish to skip this chapter. However, it is recommended that those who seek deeper and broader coverage of this background material should consult the general overview texts in the list of references.
1.2 STRUCTURE
1.2.1 CRYSTALLINE SOLIDS
Many solid materials possess an ordered internal crystal structure despite external appearances which are not what we associate with the term crystalline, i.e., clear, transparent, faceted, etc. Actual crystal structures can be imagined to arise from a three-dimensional array of points geometrically and repetitively distributed in space such that each point has identical surroundings. There are only fourteen ways to arrange points in space having this property and the resulting point arrays are known as Bravais lattices. They are shown in Fig. 1-1 with lines intentionally drawn to emphasize the symmetry of the lattice. It should be noted that only a single cell for each lattice is reproduced here and that the point array actually stretches in an endlessly repetitive fashion in all directions. If an atom or group of two or more atoms is now placed at each Bravais lattice point, a physically real crystal structure emerges. Thus, if individual copper atoms populated every point of a face-centered cubic (FCC) lattice whose cube-edge dimension, or so-called lattice parameter, were 3.54 Å, the material known as metallic copper would be generated; similarly for other types of lattices and atoms.
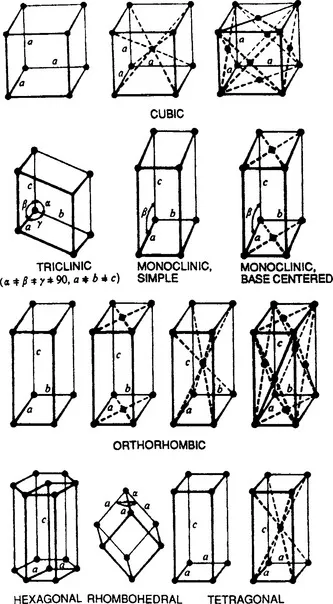
Figure 1-1 The 14 Bravais space lattices.
The reader should, of course, realize that just as there are no lines in actual crystals, there are no spheres. Each sphere in the Cu crystal structure represents the atomic nucleus surrounded by a complement of 28 core electrons [i.e., (1s)2(2s)2(2p)6(3s)2(3p)6(3d)10] and a portion of the free-electron gas contributed by 4s electrons. Furthermore, we must imagine that these spheres touch in certain crystallographic directions and that their packing is rather dense. In FCC structures the atom spheres touch along the direction of the face diagonals, i.e., [110], but not along the face edge directions, i.e., [100]. This means that the planes containing the three face diagonals shown in Fig. 1-2a, i.e., (111), are close packed. On this plane the atoms touch each other in much the same way that a racked set of billiard balls do on a pool table. All other planes in the FCC structure are less densely packed and thus contain fewer atoms per unit area. Placement of two identical silicon atoms at each FCC point would result in the formation of the diamond-cubic silicon structure (Fig. 1-2c), whereas the rock-salt structure (Fig. 1-2b) is generated if sodium–chlorine groups were substituted for each lattice point. In both cases the positions and orientation of each two-atom motif must be preserved from point to point.
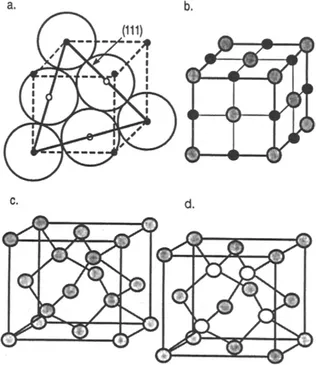
Figure 1-2 (a) (111) plane...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Foreword to First Edition
- Preface
- Acknowledgments
- Historical Perspective
- Chapter 1: A Review of Materials Science
- Chapter 2: Vacuum Science and Technology
- Chapter 3: Thin-Film Evaporation Processes
- Chapter 4: Discharges, Plasmas, and Ion–Surface Interactions
- Chapter 5: Plasma and Ion Beam Processing of Thin Films
- Chapter 6: Chemical Vapor Deposition
- Chapter 7: Substrate Surfaces and Thin-Film Nucleation
- Chapter 8: Epitaxy
- Chapter 9: Film Structure
- Chapter 10: Characterization of Thin Films and Surfaces
- Chapter 11: Interdiffusion, Reactions, and Transformations in Thin Films
- Chapter 12: Mechanical Properties of Thin Films
- Appendix
- Index