1.1 INTRODUCTION
The study of marine chemistry encompasses all chemical changes that occur in seawater and the sediments. The ocean is a place where biological, physical, geological, and chemical processes interact, making the study of marine chemistry very interdisciplinary and more appropriately termed marine biogeochemistry. Chemical approaches are now commonly used by marine biologists, marine geologists, and physical oceanographers in support of their research efforts. Likewise, oceanographers recognize the interconnectedness of Earth’s hydrosphere with its atmosphere and crust, requiring that a true understanding of the ocean include consideration of its interactions with the rest of the planet. Also important are extraterrestrial forces, such as changes in solar energy and meteorites. For these reasons, this textbook covers topics that range far beyond the margins of the seashore and seafloor, as well as the boundaries of a classical study of chemistry.
1.2 WHY THE STUDY OF MARINE BIOGEOCHEMISTRY IS IMPORTANT
Most of the water on Earth’s surface is in the ocean; relatively little is present in the atmosphere or on land. Because of its chemical and physical properties, this water has had a great influence on the continuing biogeochemical evolution of our planet. Most notably, water is an excellent solvent. As such, the oceans contain at least a little bit of almost every substance present on this planet. Reaction probability is enhanced if the reactants are in dissolved form as compared with their gaseous or solid phases. Many of the chemical changes that occur in seawater and the sediments are mediated by marine organisms. In some cases, marine organisms have developed unique biosynthetic pathways to help them survive the environmental conditions found only in the oceans. Some of their metabolic products have proven useful to humans as pharmaceuticals, nutraceuticals, food additives, and cosmeceuticals.
Another important characteristic of water is its ability to absorb a great deal of heat without undergoing much of an increase in temperature. This enables the ocean to act as a huge heat absorber, thereby influencing weather and climate.
Thus through many means, water sustains life, both marine and terrestrial. Scientific evidence supports the hypothesis that on Earth, life first evolved in a wet environment, such as an early ocean or submarine hydrothermal system. In turn, biological activity has had important effects on the chemical evolution of the planet. For example, the photosynthetic metabolism of plants is responsible for the relatively high concentration of oxygen gas (O2) in our present-day atmosphere. Most of this oxygen was originally present as CO2 emitted onto Earth’s surface as part of volcanic gases. Over the millennia, photosynthesizers, such as marine phytoplankton, have converted this CO2 into O2 and organic matter (their biomass). Burial of their dead biomass (organic matter) in marine sediments has enabled O2 to accumulate in the atmosphere. In this way, microscopic organisms have effected a global-scale transformation and transport of chemicals. This in only one example of many in which microscopic organisms serve as global bioengineers.
In studying the ocean, marine biogeochemists focus on exchanges of energy and material between the crust, atmosphere, and ocean. These exchanges exert a central influence on the continuing biogeochemical evolution of Earth. Particular concern is currently focused on the role of the ocean in the uptake and release of greenhouse gases, such as CO2. As part of the atmosphere, these gases influence solar heat retention and, hence, influence important aspects of climate, such as global temperatures, the hydrological cycle, and weather, including tropical storms. Exchanges of material between the land and sea control the distribution of marine life. For example, transport of nutrients from the nearby continents causes marine organisms to grow in greater abundance in coastal waters than in the open ocean. The exchange rates of many substances have been or are being altered by human activities. Thus, the study of marine chemistry has great practical significance in helping us learn how to use the ocean’s vast mineral and biological resources in a sustainable fashion to ensure its health for future generations of humans and other organisms.
1.3 THE CRUSTAL-OCEAN-ATMOSPHERE FACTORY AND GLOBAL BIOGEOCHEMICAL CYCLES
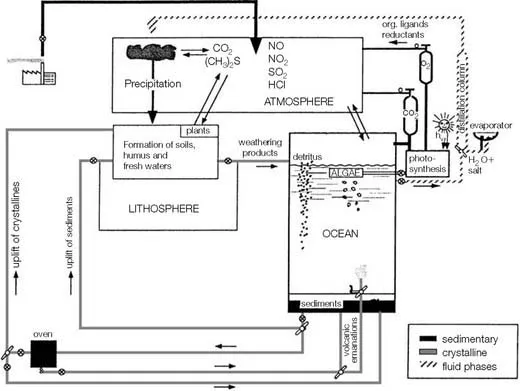
As illustrated in Figure 1.1, the planet can be viewed as a giant chemical factory in which elements are transported from one location to another. Along the way, some undergo chemical transformations. These changes are promoted by the ubiquitous presence of liquid water, which is also the most important transporting agent on Earth’s surface. It carries dissolved and particulate chemicals from the land and the inner earth into the ocean via rivers and hydrothermal vents. Chemical changes that occur in the ocean cause most of these materials to eventually become buried as sediments or diffuse across the sea surface to accumulate in the atmosphere. Geological processes uplift marine sediments to locations where terrestrial weathering followed by river transport returns the chemicals to the ocean. The mobility of chemicals within the crustal-ocean-atmosphere factory is strongly affected by partitioning at interfaces. In the ocean, these include the air-sea and sediment-water interfaces, as well as the contact zone between seawater and suspended or sinking particulate matter. Thus, the ocean acts as a giant stirred flow-through reactor in which solutes and solids are added, transformed, and removed.
The representation of the ocean presented in Figure 1.1 is not a complete description of the ocean but serves to illustrate aspects important to the ...