
- 731 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Combustion, Flames and Explosions of Gases
About this book
Combustion, Flames and Explosions of Gases, Third Edition provides the chemist, physicist, and engineer with the scientific basis for understanding combustion phenomena.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Combustion, Flames and Explosions of Gases by Bernard Lewis,Guenther von Elbe in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Chemical & Biochemical Engineering. We have over one million books available in our catalogue for you to explore.
Information
Edition
3Part I
Chemistry and Kinetics of the Reactions between Gaseous Fuel and Oxidants
CHAPTER I
Theoretical Foundations
Publisher Summary
This chapter describes the systems of gases that are capable of spontaneously accelerating chemical reaction with large energy release. In a volume of such gas or gas mixture, the reaction may occur more or less simultaneously throughout the volume. The chapter also discusses some fundamentals of reaction kinetics. The molecules of a gas are in rapid motion and collide frequently with each other. The number of collisions that occurs in a gas per unit time and volume between two kinds of molecules is proportional to the concentration of each molecule. The chapter develops equations for free-radical reaction rates and explosion limits. It presents the general case of isothermal branched-chain reactions with chain breaking at the vessel surface.
1 Free-Radical Chain Reactions
This book deals with systems of gases that are capable of spontaneously accelerating chemical reaction with large energy release. In a volume of such gas or gas mixture the reaction may proceed as a wave propagating from a localized source of ignition, or it may occur more or less simultaneously throughout the volume. In this and the chapters immediately following we consider principally the latter mode of reaction.
The molecules of a gas are in rapid motion and collide frequently with each other. The chance for chemical reaction between two colliding molecules is largely dependent on temperature according to the Arrhenius function e−E/RT, where R = 1.987 cal/mole, °K is the gas constant, T is the absolute temperature, and E is the activation energy. The latter represents the minimum energy that must be possessed by the colliding molecules in order to effect chemical change. Owing to the high bond energies of the atoms, the activation energies required in collisions of, for instance, H2 and O2 or H2 and Cl2 are so large that mixtures of these gases do not react perceptibly at ordinary temperatures over long periods, even though a molecule suffers about a billion collisions per second at atmospheric pressure. Any such mixture of gaseous fuel and oxidant tends, however, to become destabilized by the addition of free radicals, which are molecular species that possess free chemical bonds. They include free atoms such as H or Cl each of which possesses one free bond, and oxygen atoms, O, which possess two free bonds.
For example, the reaction
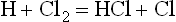
has an activation energy of no more than about 4000 cal/mole1 and accordingly occurs at ordinary temperature (300°K) once in about a thousand collisions between H and Cl2. Also, the reaction
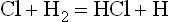
has an activation energy of no more than about 6000 cal/mole, which gives it a chance of occurring once in roughly ten to twenty thousand collisions of Cl and H2. With collisions occurring on the order of billions per second, the two reactions will follow each other in rapid sequence and produce about 106 molecules of HCl per second at ordinary temperature and pressure.
This is an example of a free-radial chain reaction. In this type of reaction a free radical that is generated from a fuel molecule (e.g., H from H2) reacts with an oxidant molecule (e.g., Cl2) to form a product molecule (e.g., HCl) and an “oxidant” free radical (e.g., Cl), which in turn reacts with a fuel molecule to form a product molecule and to regenerate a “fuel” free radical, and so on.
In the present example, one of the mixture components is chlorine which absorbs light over a wide spectral range and generates chlorine atoms by photodissociation according to
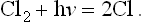
Thus, illumination of a transparent reaction vessel containing H2 and Cl2 generates two reaction chains for each absorbed light quantum hv. It follows that a moderate flash of light corresponding to, say, 1012 light quanta hv absorbed per cubic centimeter, would at ordinary gas temperature and pressure initiate the formation of HCl at a rate of roughly 1018 molecules/cm3 sec. Heat would be generated according to the equation
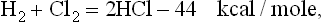
and, depending on various system parameters including the size of the reaction vessel, an imbalance might develop in which more heat is generated than is dissipated to the environment. Because of this the temperature rises, the reaction rate increases and thermal explosion results. Such explosions of hydrogen-chlorine mixtures by exposure to light have been experienced voluntarily and involuntarily in many laboratories and classrooms.
If the various free-radical species of a fuel–oxidant system each carry only one free bond as is the case in the present example, the reaction chain comprises merely a series of free-bond transfers from a fuel free radical to an oxidant free radical and back to a fuel free radical without producing any additional free bonds. But if one substitutes O2 for Cl2 and writes analogously
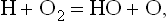
one obtains two oxidant free radicals that carry a total of three free bonds, or two free bonds in addition to the free bond initially possessed by the hydrogen atom. This is called chain bra...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface to the Third Edition
- Preface to the Second Edition
- Preface to the First Edition
- List of Principal Symbols
- Part I: Chemistry and Kinetics of the Reactions between Gaseous Fuel and Oxidants
- Part II: Flame Propagation
- Part III: State of the Burned Gas
- Part IV: Technical Combustion Processes
- Appendixes
- Author Index
- Subject Index